Age-related Yields of Mouse Sperm and Oocytes
Michael Ramin, Tim Holland-Letz, Anna Schwab, Johannes Schenkel
DOI10.21767/2572-5459.100022
Michael Ramin1, Tim Holland-Letz1, Anna Schwab1 and Johannes Schenkel1,2*
1German Cancer Research Center (DKFZ), Cryopreservation W430, Heidelberg, Germany
2Institute of Physiology and Pathophysiology, University, Heidelberg, Germany
- *Corresponding Author:
- Schenkel J
German Cancer Research Center (DKFZ), Cryopreservation W430 Im Neuenheimer Feld 280
D-69120, Heidelberg, Germany
Tel: +49-6221-423350
Fax +49-6221-423209
E-mail: j.schenkel@dkfz.de
Received date: January 09, 2017; Accepted date: March 1, 2017; Published date: March 3, 2017
Citation: RaminM, Holland-Letz T, Schwab A, Schenkel J (2017) Age-Related Yields of Mouse Sperm and Oocytes. J Anim Res Nutr. 2:2. doi: 10.21767/2572-5459.100022
Copyright: © 2017 Ramin, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Title: Genetically modified animals, in particular mice, are unique mutants with a major scientific potential. Cryopreservation of pre-implantation embryos or of spermatozoa is a common approach to protect those lines against loss or to archive them. Prerequisite to take a mutant line out of a breeding nucleus is the cryopreservation of a sufficient number of samples and a powerful quality assessment.
Background: Several common protocols to cryopreserve spermatozoa and subsequently to forward these to an in vitro-fertilization under standardized conditions are published using donors of a strictly limited age-span. In practice, several limitations happen frequently for those approaches, e.g. the availability of genetically modified males, in particular if a line is in an experimental use or the mutation leads to restrictions. Female donors are often received from external sources and are subsequently not available before weaning. They are subject of several delays to induce an early superovulation. The aim of this report is to study the suitable age span of sperm and oocyte donors.
Methods and Findings: A total of 441 GM sperm donors and the production of wild type oocytes with the genetic backgrounds BALB/c, C57BL/6N, and DBA/2 were investigated. The quality was assessed with simple, but purposive scores. Polynomial statistical analyses investigating increasing and decreasing yields show that the age of sperm-donors can be extended to ten months; the suitable age of oocyte donors is strain specific up to twelve weeks.
Conclusions: The extended age spans demonstrated allow a more flexible use of donors and contribute subsequently to the 3Rs.
Keywords
Cryopreservation; Genetically modified (GM) mice; 3Rs; Quality assessment; Scoring
Introduction
In basic research genetically modified (GM) animals are unique mutants to study genes, their function, regulating mechanisms, (human) diseases, or others. GM mouse lines have an enormous (scientific) value and must be protected against loss or unexpected disasters. Furthermore, they should remain available for the future even if they are out of a current use. Cryopreservation of pre-implantation embryos or of spermatozoa is a powerful tool and a common approach to save and handle the increasing number of GM mouse lines, often bad breeding and consisting of just very small populations. Only after a successful and powerful assessment of cryopreserved specimens a line can be taken out of a breeding nucleus [1,2]. The cryopreservation procedure itself, high yields in the production of spermatozoa, oocytes, or embryos as well as new assessment strategies result in a significant reduction of the number of laboratory animals used for the preservation of a line [1] and contribute subsequently to the 3Rs [3].
In recent years the cryopreservation of mouse spermatozoa was improved to become a reliable tool [4-6]. However, an in vitro-fertilization required to recover a line from frozen spermatozoa is complex [7,8] the success is often influenced externally, e.g. genetic background, mutation, osmotic stress, batches of the culture media used, environment, etc. [4-6,9-11].
In practice, the use of an in house-cryopreservation facility has been proven to keep several advantages, especially a particular flexibility and a possibly multiple use of a (mutant) animal, also with respect to the 3Rs [3]. This is a big advantage in case of bad breeding GM lines, too. Subsequently, donor animals can be faced to cryopreservation depending on their availability. Therefore, an extended age span of those animals might be necessary, more as recommended elsewhere, i.e. three to six months (or even less) in case of male donors [4,6,12]. Due to the biological development sperm isolation from male mice younger than three months does not make sense.
On the other hand, female donors should be subjected to superovulation and oocyte production as early as possible in their puberty. In case of animals purchased from external sources, this is conflicting with some restrictions on availability e.g., the date of shipment (de facto possible just after weaning), and the adaptation in the new facility, etc., i.e. it remains impossible to meet the optimum age for superovulation. For investigations with females before the physiological induction of their first genital cycle it is sometimes recommended to breed those animals in in house-animal facilities [12]. However, this is often not feasible. Several superovulation protocols were published, in general dealing with donor animals at an optimum age [12-15].
In this study, we tried to understand better the possible donor age limits for those purposes in order to combine animal welfare, high yields, and the given circumstances in the day-today operations of a laboratory. The quality of the sperm was assessed by using a simple scoring approach.
Materials and Methods
Animals and housing
Mice were housed in the animal facility of the German Cancer Research Center, Heidelberg, Germany (DKFZ). GM mice were bred in house, whereas wild type (WT) mice on a common genetic background (BALB/c, C57BL/6N, DBA/2) were received from commercial breeders in an age of 21 to 23 days (Charles River Germany, Sulzfeld Germany). All animals were fed with phytoestrogen-poor diet [16] ad libitum. The age of the males ranged between three and ten months, the genetic background was in the majority C57BL/6 with an unknown number of backcross generations, males on other genetic backgrounds (BALB/c, DBA/2, BDF-hybrids) were also investigated.
Individually ventilated caging systems (IVC) and specified pathogen free (SPF) facilities (barrier, with open caging systems) were used as described earlier in detail [1]. Males were kept singly and females in groups of five animals. Health monitoring of the animals was performed according to the FELASA recommendations [17]. Most mice were housed in an IVC-facility which was started to run under SPF-conditions, within the time of this study no infectious agents were detected as listed by the FELASA guidelines.
Animal experimentation was in agreement with the German animal welfare act. All animal experiments were licensed by the animal welfare department of the competent authority (Regierungspräsidium Karlsruhe, Germany) and under the surveillance of the intramural animal welfare committee.
Sampling of spermatozoa
Spermatozoa were prepared according to [1,4] and afterwards used for cryopreservation or subjected directly to in vitrofertilisation (IVF). These procedures were for all donors the same, independent on the genetic background. In brief, males were sacrificed by cervical dislocation; epididimydes and vasa deferentia were prepared. Spermatozoa were collected following several incisions in the epididymides and squeezing out any sperm from the vasa deferentia. Spermatozoa were allowed to disperse from the tissue for 10 min at 37°C in cryoprotective media (CPM: 18% [w/v] raffinose [Sigma-Aldrich], 3% [w/v] skim milk [BD diagnostics], and 477 μM monothioglycerol [Sigma-Aldrich] in distilled water). Samples for quality assessments were taken from the rim of the preparation drop. Spermatozoa in CPM were loaded into 0.25 mL French straws (IMV Technologies, Rambouillet, France). Straws were sealed with an impulse tong sealer (Polystar 110 GE/150 D; Rische & Herfurth, Hamburg, Germany), placed onto a polystyrene raft floating in liquid nitrogen (LN2) at least for 10 min and then stored in LN2. Accordingly, after thawing cryopreserved spermatozoa were forwarded into COOKmedium, (Cook Medical Ltd, Eight Mail Plains, Australia) incubated for 1 h and afterwards assessed as described above.
Scoring of spermatozoa
The motility was investigated with samples taken from the rim of the micro-drops and light-microscopically scored. All spermatozoa of a field of view were scored as “0” (no motility), “1” (slow forward motility), “2” (partial forward motility) to “3” (high forward motility). Accordingly, a sperm with a high circular but not forward motility was scored as “1”. Out of these, the percentage of moving a spermatozoon was estimated and accordingly the motility score was reduced, a simplified procedure according to [8,18,19]. E.g., in a sample with a very high forward motility just 75% of all spermatozoa were moving the score of “3” was reduced to 75%, i.e. 2.25. Similarly, the density was microscopically scored: from “0” no cells, “1” a few cells (about 0.1 × 106 cells/ml), “2” one microscopically detectable layer of cells (about 1 × 106 cells/ml), and “3” several layers of spermatozoa (about 10 × 106 cells/ml). The cellnumbers are calculated for the concentration in the original samples, i.e. all dilution steps are included. Cross-checks by two experienced persons lead to the same result.
Sampling of oocytes
The (first) genital cycle of pubertal donor females was induced by superovulation as described elsewhere [12,20]. 7 IU of both hormones were injected in an appropriate volume into C57BL/6N mice, in case of BALB/c and DBA/2 5 IU each was used. These animals were sacrificed 12 to 14 h after the application of human Chorionic Gonadotropin and the oocytes were prepared from the swollen ampullas. For the investigations described here the in vitro-fertilization media under oil were used according to Ostermeier et al. [4]. To show the oocyte yield, after five to six hours of co-incubation, the presumptive zygotes were washed and counted.
Statistics
The outcome of our experiments in regard to both the number of oocytes and the motility of spermatozoa depending on the age of the donors was described using linear regression models. As we expect an initial increase of yields with age of donor passing over into an actual decrease at larger age values, the relationship cannot be adequately modelled using a simple linear relationship. Instead, a better data fit can be achieved using a polynomial of the second order. In this model, a positive linear term represents the general increase in yields, while a negative quadratic term can still predict the late term decrease in results. Differences between means were considered significant with a p<0.05, and trending towards significance with a p<0.10. The coefficient of determination indicates how well the model fit the data, R²=0 indicates no fit, R²=1 indicates a perfect fit. All calculations were done with SigmaPlot (Systat Software Inc., Erkrath, Germany).
Results
Spermatozoa
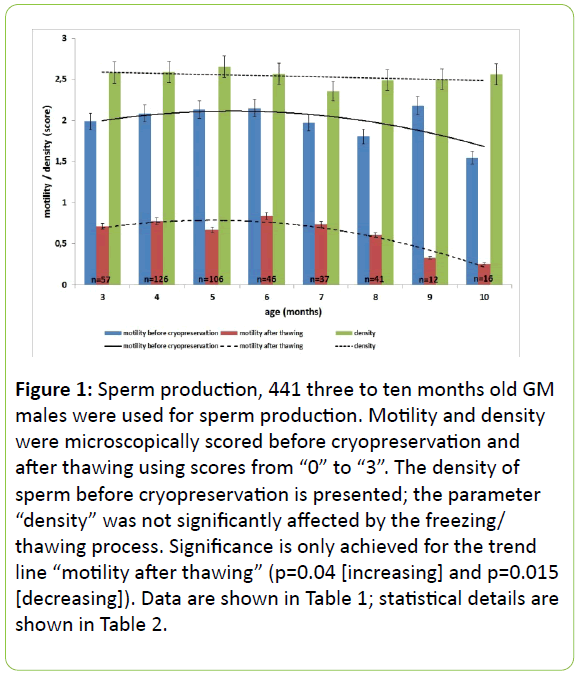
To elucidate whether the published suitable age span of sperm-donor mice from three to six months might have the capacity to become extended, the yield of spermatozoa was investigated under respect of the age of the donors. 441 mutant male mice between three and ten months of age served as sperm-donors (Table 1 and Figure 1). The animals selected were part of running cryopreservations within seven years. Only animals of mutant lines without any obvious influence of the mutation on their reproductivity were introduced into this study. The polynomial analysis showed in general that just the motility of frozen/thawed spermatozoa is significantly correlated with the age of the donor (Table 2). Accordingly, the correlation of increasing motility of frozen/thawed sperm with the increasing age is significant. The decrease of the motility of frozen/thawed sperm calculated with the formula using the quadratic age shows also significance. Subsequently, the calculated coefficient of determination of the polynomial trend lines was high for frozen/thawed freshly prepared sperm and rather low for the other parameters (Table 2). Taken together, a small peak in the motility was detected at a donor age of six months but no major age-effects were observed in the time-span investigated (three to ten months). Afterwards, the quality will drop. All specimens were investigated before cryopreservation and after the freezing/thawing process. The parameter “density” was neither affected by the cryopreservation and recovery process (data not shown in detail) nor by the age of the donors. Table 1 shows the assessment data, Table 2 the statistical analysis. The behaviour of the spermatozoa of all donors was similar.
| Age of donor (months) | # of donors | % Motility before cryo | % Motility frozen/thawed | Density before cryo | Density frozen/thawed |
|---|---|---|---|---|---|
| 3 | 57 | 1.99 | 0.71 | 2.58 | 2.56 |
| 4 | 126 | 2.08 | 0.77 | 2.59 | 2.54 |
| 5 | 106 | 2.13 | 0.67 | 2.65 | 2.61 |
| 6 | 46 | 2.15 | 0.84 | 2.57 | 2.65 |
| 7 | 37 | 1.97 | 0.73 | 2.35 | 2.46 |
| 8 | 41 | 1.80 | 0.60 | 2.49 | 2.56 |
| 9 | 12 | 2.18 | 0.33 | 2.50 | 2.42 |
| 10 | 16 | 1.54 | 0.25 | 2.56 | 2.38 |
Table 1: Spermatozoa production and assessment, age and number of spermatozoa donors and sperm assessment. Motility-scores: “0” (no motility), “1” (slow forward motility), “2” (partial forward motility) to “3” (high forward motility). Density-scores: “0” no cells, “1” a few cells, “2” one microscopically detectable layer of cells, and “3” several layers of spermatozoa.
Figure 1: Sperm production, 441 three to ten months old GM males were used for sperm production. Motility and density were microscopically scored before cryopreservation and after thawing using scores from “0” to “3”. The density of sperm before cryopreservation is presented; the parameter “density” was not significantly affected by the freezing/ thawing process. Significance is only achieved for the trend line “motility after thawing” (p=0.04 [increasing] and p=0.015 [decreasing]). Data are shown in Table 1; statistical details are shown in Table 2.
| Motility before cryopreservation | Motility of frozen/thawed spermatozoa | Density | ||||
|---|---|---|---|---|---|---|
| Correlation | p=0.192 | p=0.004 | p=0.43 | |||
| coefficient | p-value | coefficient | p-value | coefficient | p-value | |
| Intercept (beta0) | 1.52 | p=0.041 | 0.215 | p=0.423 | 2.87 | p<0.001 |
| Linear effect of Age (beta1) | 0.219 | p=0.290 | 0.227 | p=0.040 | -0.098 | p=0.331 |
| Quadratic effect of age (beta2) | -0.02 | p=0.208 | -0.023 | p=0.015 | 0.006 | p=0.399 |
| R2 | 0.4828 | 0.8955 | 0.1646 | |||
Table 2: Statistical analysis of spermatozoa, the table shows the polynomial regression model used to model the dependence between age and motility/density. The density of samples before the cryopreservation process is presented; the parameter “density” was not significantly affected by the freezing/thawing process. The linear effect beta1 models the general increase with age while the quadratic effect beta2 represents a possible decrease for larger age values. The R2 value describes the overall fit of the model. The predictive formula can be used to predict the expected motility/density for any given age value. Predictive Formula: Motility/Density=beta0+ beta1*age+beta2*age^2.
Oocytes
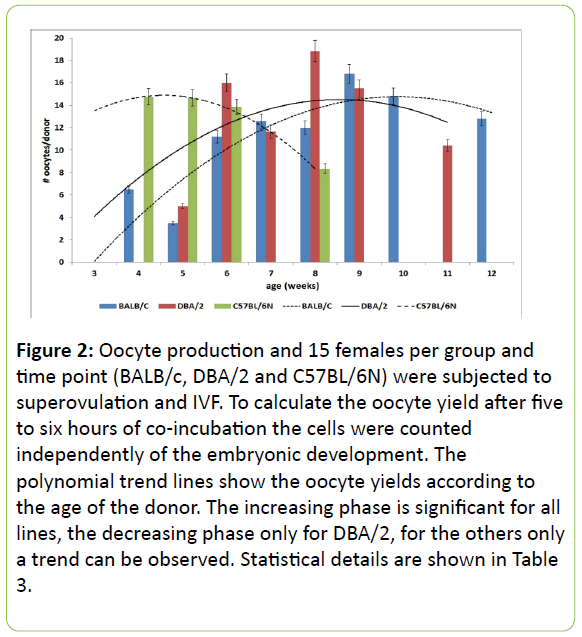
To determine a satisfying age span of donor mice on commonly used genetic backgrounds for the production oocytes, the yield of oocytes was investigated for the wild type strains BALB/c, DBA/2, and C57BL/6N. 15 females per group and time point were super ovulated, the oocyte-cumulus cell complex was prepared as described. Due to experimental reasons the number of oocytes was calculated following coincubation with spermatozoa and afterwards analysed statistically. The age of the donors ranged between three and twelve weeks, details are shown in Table 3 and Figure 2. Younger females did not lead to any oocyte production. The age of the donor and the oocyte yield are significantly (C57BL/6N and DBA/2) or highly significant (BALB/c) correlated. To understand the role of the donor age better, the correlation between increasing age and increasing oocyte-yield was highly significant (BALB/c), significant (DBA/2), or a trend was determined (C57BL/ 6N). The decrease of the yields calculated with the formula using the quadratic age showed significance for DBA/2 and a trend for BALB/c and C57BL/6N. The coefficients of determination of the polynomial trend lines were calculated as R²>0.6707, see Table 4. Peaks of yields were found at 28 d (C57BL/6N), 56 d (DBA/2), and 70 d (BALB/c), the range of a suitable age of the donors is strain specific (Figure 2).
| Age of oocyte donor (weeks) | # of Oocytes/donor BALB/c |
# of Oocytes/donor DBA/2 |
# of Oocytes/donor C57BL/6 N |
|---|---|---|---|
| 4 | 6.5 ± 5.55 | nd | 14.75 ± 4.78 |
| 5 | 3.5 ± 1.73 | 5 ± 3.46 | 14.66 ± 4.63 |
| 6 | 11.2 ± 6.01 | 16 ± 7.55 | 13.85 ± 9.28 |
| 7 | 12.6 ± 5.36 | 11.66 ± 9.52 | nd |
| 8 | 12 ± 9.6 | 18.83 ± 7.96 | 8.33 ± 3.51 |
| 9 | 16.8 ± 4.08 | 15.5 ± 8.76 | nd |
| 10 | 14.8 ± 6.9 | nd | nd |
| 11 | nd | 10.4 ± 2.88 | nd |
| 12 | 12.8 ± 8.4 | nd | nd |
Table 3: Oocytes production, Donor age and number of oocytes/donor (nd=not done)
| BALB/c | DBA/2 | C57BL/6N | ||||
|---|---|---|---|---|---|---|
| n per time point | 15 | 15 | 15 | |||
| time points | 9 | 4 | 4 | |||
| Correlation | p<0.001 | p=0.037 | p=0.031 | |||
| coefficient | p-value | coefficient | p-value | coefficient | p-value | |
| Intercept (beta0) | -6.09 | p=0.056 | -26.3 | p=0.055 | 3.21 | p=0.302 |
| Linear effect of age (beta1) | 3.52 | p=0.007 | 10.2 | p=0.026 | 5.10 | p=0.071 |
| Quadratic effect of age (beta2) | -0.15 | p=0.083 | -0.62 | p =0.041 | -0.56 | p =0.053 |
| R2 | 0.8738 | 0.6707 | 0.999 | |||
Table 4: Statistical analysis of the oocyte production, the table shows the polynomial regression model used to model the dependence between age and number of oocytes. The linear effect beta1 models the general increase with age while the quadratic effect beta2 represents a possible decrease for larger age values. The R2 value describes the overall fit of the model. The predictive formula can be used to predict the expected number of oocytes for any given age value. Predictive Formula: Number of Oocytes=beta0+ beta1*age+beta2*age^2.
Figure 2: Oocyte production and 15 females per group and time point (BALB/c, DBA/2 and C57BL/6N) were subjected to superovulation and IVF. To calculate the oocyte yield after five to six hours of co-incubation the cells were counted independently of the embryonic development. The polynomial trend lines show the oocyte yields according to the age of the donor. The increasing phase is significant for all lines, the decreasing phase only for DBA/2, for the others only a trend can be observed. Statistical details are shown in Table 3.
Discussion
Cryopreservation is a valuable and important tool when dealing with mutant mice. However, all cryopreservation and recovery processes are animal consuming and especially under respect of the animal welfare and the 3R potentials to reduce the number of animals for these purposes should be investigated. Aim of this study was to elucidate the suitable range of the age of female and male donors, not to investigate or compare different IVF-protocols. Many animal facilities are relying on the import of female donors from commercial breeders resulting in a delay to induce an early superovulation [12,21,22]. On the other hand, the availability of male donors is, especially in case of mutants, often very short. Several publications describe a strict time span from three to six months of donor age or less for sperm production [4,5,12]. In the everyday practice of a laboratory working with mutant mice, males have frequently to serve for other purposes before they will be finally faced to sperm donation. Subsequently we studied possibly extended time spans in the age of the female and male donors might be acceptable for the production of oocytes and sperm.
When investigating 441 mutant males out of running cryopreservation projects (males without any phenotype), in majority on a C57BL/6 genetic background, a few were on BALB/c, DBA/2, or BDF-hybrid background, we understood that the age of sperm donors can be extended up to ten months, afterwards the motility drops significantly (Figure 1, Table 2).
This was independent on the genetic background. Possibly, the viscosities of the different media used before and after thawing might slightly influence the motility. But this does not affect the general outcome of this study showing that the spermatozoa-production is parallel to the time course of the donor age. The motility is a major parameter for sperm assessment [1,7,11]. As expected (and as control), the density was never significantly affected by the age of the donor or the cryopreservation and recovery process. The simple scoring tool allows the investigation giving a quick statement about the quality of the material. To exclude additional effects und to keep the comparability upright only the media and procedures of one of the common, published approaches to cryopreserve and recover mutant lines [4] were investigated. This approach works in our hands successfully delivering similar results as other procedures [5,6]. Expensive experiments as IVF followed by embryo transfer etc. were performed only to assess the whole procedure.
In our hands the optimum time point to superovulate those females varies from strain to strain between three and nine weeks of age (Figure 2, 4). To get high yields of oocytes these data keep the opportunity to co-ordinate shipment after weaning, adaptation phase, and strain specific superovulation at optimum ages. It remains noteworthy that the optimum age for superovulation varies from strain to strain and must be investigated individually. Our data agree in part with data published elsewhere, however these publications are focussed on optimized conditions [12,13], this study tried to find acceptable limits in the day-to-day practice when dealing with those animals.
It is to be pointed out that the statistical analyses used here describe an increase and afterwards a decrease of yields. Due to this non-linear course of the data investigated, the course of correlation should not be used for an extrapolation over the time span elucidated.
It is important to note that the health status of the donors must be excellent. Some investigations with oversized, but noninfected uteri showed a dramatic drop of oocyte yields (data not shown in detail). The reasons of this hyperplasia remains unclear, it might depend on environmental factors as the diet used [16].
Taken together, the suitable age span of oocyte donors for superovulation is strain-specific. Male mice keep the capacity to be used for other purposes before serving as a sperm donor. This makes experimentation in practice easier and contributes to the 3Rs. More complex future investigations should also assess the outcome of pups following in vitro-fertilisation and embryo transfer.
Acknowledgement
The authors thank Beatrix Imkeit, Helmut Eskerski, and Andrea Rausch for expert technical assistance. There are no conflicts of interests.
References
- Diercks AK, Schwab A, Bürgers HF, Schenkel J (2012) Improved assessment of frozen/thawed mouse spermatozoa by using fluorescence microscopy. J Vet Sci13: 315-322.
- Ramin M, Bürger A, Hörlein A, Kerkau D, v. Walcke-Wulffen V, et al. (2014) Stability of cryopreserved samples of mutant mice. Biopreserv Biobank 12: 343-350.
- Russell WMS, Burch RL (1959) The Principles of humane Experimental Technique. London: Methuen & Co Ltd.
- Ostermeier GC, Wiles MV, Farley JS, Taft RA (2008) Conserving, distributing and managing genetically modified mouse lines by sperm cryopreservation. PLoS One 3: e2792.
- Takeo T, Nakagata N (2010) Combination medium of cryoprotective agents containing L-glutamine and methyl-β cyclodextrin in a pre-incubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab Anim 44: 132-137.
- Takeo T, Nakagata N (2011) Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-Beta-cyclodextrin. Biol Reprod 85: 1066-1072.
- Sztein JM, Farley JS, Young AF, Mobraaten LE (1997) Motility of Cryopreserved Mouse Spermatozoa Affected by Temperature of Collection and Rate of Thawing. Cryobiology 35: 46-52.
- Songsasen N, Betteridge KJ, Leibo SP (1997) Birth of live mice resulting from oocytes fertilized in vitro with cryopreserved spermatozoa. Biol Reprod 56: 143-152.
- Danekar PV, Glass RH (1987) Development of mouse embryos in vitro is affected by strain and culture medium. Gamete Res 17: 279-285.
- Ho Y, Wigglesworth K, Eppig JJ, Schultz RM (1995) Preimplantation development of mouse embryos in KSOM: Augmentation by amino acids and analysis of gene expression. Mol Reprod Dev 41: 232-238.
- Walters EM, Men H, Agca Y, Mullen SF, Critser ES, Critser J (2005) Osmotic tolerance of mouse spermatozoa from various genetic backgrounds: Acrosome integrity, membrane integrity, and maintenance of motility. Cryobiology 50: 193-205.
- Behringer R, Gertsenstein M, Vintersten-Nagy K; Nagy A (2014) Manipulating the mouse embryo: A laboratory manual 4th ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Byers SL, Payson SJ, Taft RA (2006) Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs). Theriogenology 65(9):1716-1726
- Takeo T, Nakagata N (2015) Superovulation using the combined administration of inhibin antiserum and equine chorionic gonadotropin increases the number of ovulated oocytes in C57BL/6 female mice. PLoS One 10: e0128330.
- Vasudevan K, Sztein JM (2012) In vitro fertility rate of 129 strain is improved by buserelin (gonadotropin-releasing hormone) administration prior to superovulation. Lab Anim. 46: 299-303.
- Ramin M, Denk N, Schenkel J (2015) The role of diet and housing-temperature in the production of genetically modified mouse embryos and their developmental capacity after cryopreservation. Theriogenology 84: 1306-1313.
- Mähler M, Berard M, Feinstein R, Gallagher A, Illgen-Wilcke B, et al. (2014)FELASA working group on revision of guidelines for health monitoring of rodents and rabbits. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim 48: 178-192.
- Jequier AM, Crich JP (1986) Semen Analysis: A Practical Guide. Oxford, UK: Blackwell Publications 56-62.
- Aral F, Karaçal F, Baba F (2008) The effect of enrofloxacin on sperm quality in male mice. Res Vet Sci 84: 95-99.
- Schwab A, Schenkel J (2008) Collection, Cryopreservation, Storage And Revitalization Of Transgenic Mouse Embryos. Cold Spring Harb Protoc 3: 1215-1223.
- Gates AH (1971) Maximizing yield and developmental uniformity of eggs, methods in mammalian embryology, San Francisco, Cal, Freeman 64-75.
- Kolbe T, Na E, Urban I, Michel G (2015) Productivity of superovulated C57BL/6J oocyte donors at different ages. Lab Anim (NY) 44: 346-349.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences