Effect of Cross-Fostering on a Variety of Viral Agents in Conventionally Bred Rats
Karaman M, Baltacıoglu G, Kolatan HE, Celik A, Yilmaz O, Guneli E
DOI10.21767/2572-5459.100032
Karaman M1,2*, BaltacÃÆââ¬Å¾Ãâñoglu G1, Kolatan HE1, Celik A1, Yilmaz O1 and Guneli E1
1Department of Microbiology and Clinical Microbiology, Central Laboratory of Animal Science, Dokuz Eylul University Institute of Medical Sciences, Izmir, Turkey
2Department of Medical Microbiology, Dokuz Eylul University Faculty of Medicine, Izmir, Turkey
- *Corresponding Author:
- Meral Karaman
Department of Microbiology and Clinical Microbiology
Dokuz Eylul University Hospital, Central Laboratory of Animal Science
35340 Izmir-Turkey
Tel: 90 232 4124517
Fax: +90 232 4129798
E-mail: meral.karaman@deu.edu.tr
Received date: July 21, 2017; Accepted date: August 11, 2017; Published date: August 16, 2017
Citation: Karaman M, BaltacÃÆââ¬Å¾Ãâñoglu G, Kolatan HE, Celik A, Yilmaz O, et al. (2017) Effect of Cross-Fostering on a Variety of Viral Agents in Conventionally Bred Rats. J Anim Res Nutr Vol. 2 No. 2:12 doi: 10.21767/2572-5459.100032
Copyright: © 2017 Karaman M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
We aimed to compare the serological profiles of the pups that were housed with their biological mothers and the ones which were transferred to foster-dams after birth, with regard to certain viral agents. The day of delivery for the pregnant rat is recognized as the postnatal day 0 (P0). The pups born from Group 1 [P(0), P(21), P(60)] pregnant rats were housed with their biological mothers. In Group 2 [P(1), P(21), P(60)], however, P(1) consisted of pups housed with their biological mother for 24 hours while P(21) and P(60) consisted of pups transferred from the their biological mother immediately at birth. Antibody response against Lymphocytic Choriomeningitis Virus (LCMV), Rat Parvo Virus (RPV), Sendai Virus (SV) and Toolan's H1 viruses was not detected in dams and pups of all the groups, whereas high rate (45%) of antibody positivity was observed in response to Kilham' Rat Virus (KRV). No statistically significant difference was determined between the pups left with their biological mother and with the foster mothers, except for Murine Adeno Virus type 1, type 2 (MAD 1 and 2), Reovirus type 3 (REO-3) and Theiler's Murine Encephalomyelitis Virus (TMEV). It was conspicuous in terms of REO-3 that for the pups it was essential to stay with their biological mothers and nursed during the first 24 hours. As a result, foster mother usage in breeding laboratory animals is found to be beneficial against a variety of viral agents for the pups particularly in the later period of their lives and we suggest to continue this implementation in routine laboratory practice whenever necessary.
Keywords
Cross-fostering; Rat; Microbiological monitoring; Viral agents
Introduction
Experimental animals are widely used in many fields such as infectious, immunologic and endocrinological diseases and cancer ethiopathogenesis, and they provide important data for the protection of human health and quality of life. It is essential for the experimental research animals (laboratory animals) to be healthy and showing no signs of any clinical disease as well as carrying no infectious agent in latent form. Microbiological agents are known to have considerable impact on the health status of laboratory animals and on the outcomes of the experimental studies. Physiological, biochemical and endocrinological parameters of sick and healthy animals are normally different from each other so these may affect the outcomes of the experimental studies [1-3].
Rats are among the most commonly used animals for scientific purposes [4]. Rat pups are born hairless and with eyes closed, after a gestation period of 20-22 days. They acquire passive immunity from their mothers through placenta and colostrum which protect them against various infectious agents in the first few days of their lives [5,6]. Therefore it is very important to keep rat pups with their biological mother’s right after birth and allow for nursing. Fostering is routinely used in breeding of both laboratory and farm animals, and it is important for supporting pups whose dams die in lactation period or while giving birth to a bigger pup.
In laboratory animal science, protective medicine is fundamental. It is important to observe the symptoms and determine any disease and take precautions before it spreads to the colony. It is also important to have information about the microbiological status of the experimental animals and their environment with a view to animal welfare too, in addition to reliable scientific results. For this purpose, the Federation of European Laboratory Animal Science Associations (FELASA) is regularly revising its recommendations on monitoring the health of the production colonies or experimental units [7].
In this study, we investigated the effects of cross-fostering on microbiological monitoring of conventionally bred rats. We aimed to determine the serological profiles of the pups that were either housed with their biological mothers or transferred to foster-mothers and compare these profiles against certain viral agents that can be seen in the laboratory animal facilities and as well as to demonstrate potential differences between them.
Materials and Methods
Animals
Conventionally bred and housed rats were obtained from the Multidisciplinary Animal Laboratory of Dokuz Eylul University (DEU). The laboratory has authorization within the framework of the regulation issued by Food, Agriculture and Livestock Ministry framework since 2008 for the production, use and supply of animal species such as mice, rats, guinea pigs and rabbits. The study was approved by the Ethics Committee of the Research of Laboratory Animals, DEU Medical School. The rats were housed in hygienic makrolon cages, with ad libitum-feeding, in airconditioned rooms with 12- hour light-dark cycle. The protocols complied with the standards in the Guide for the Care and Use of Laboratory Animals, prepared by the National Academy Press, National Research Council [8].
Study groups
Nonsibling and virgin Wistar albino female rats (200-230g) (n=6) were mated in separate cages. (Power analysis was performed to use the minimum number of animals.) Following mating, positively pregnant females were marked and kept in separate cages under standard conditions until delivery. The pregnant females comprised of randomly grouped and blood samples were collected to determine their baseline serological status. All the females gave birth in two days. First four females were taken to biological mother group, other two females taken to foster mother group. Pups of foster mothers were taken to stock colony. The day of delivery was recognized as the postnatal day 0 P(0). Considering postnatal period, Group 1 was subdivided into P(0), P(21), P(60) subgroups and Group 2 into P(1), P(21), P(60) subgroups. Groups and characteristics are summarized in Table 1. The dams and pups in all groups were weighed and recorded. In case the mother gave birth to more than seven litters, then these were excluded from the study and transferred to breeding colony. We made no discrimination between female and male pups born in the study. After completion of the experimental protocol described in Table 1, dams and pups in each group were sacrificed by cervical dislocation and then the blood samples were collected by cardiac exsanguinations [9]. Sera were separated and stored at -80°C until the day of testing.
Determination of viral antibodies by ELISA method
On the test day, rat serum samples were taken out of the freezer at -80°C and left to dissolve at +4°C. The levels of antibodies against Kilham’s Rat Virus (KRV), Lymphocytic Choriomeningitis Virus (LCMV), Murine Adeno Virus Type 1, Type 2 (MAD 1 and 2), Rat Parvo Virus (RPV), Reovirus Type 3 (REO-3), Sendai Virus (SV), Sialodacryoadenitis Virus / Rat Coranavirus (SDAV/RCV), Toolan’s Virus (H1), Theiler’s Murine Encephalomyelitis Virus (TMEV) were tested by ELISA method in accordance with the manufacturer's recommendations (SMART, Biotech Trading Partners, CA-USA). The optical density values of the plates were measured by an ELISA microplate reader device (Thermo Electron Corporation, Varioskan Flash, USA) at 450/620-650 nm. Index value was calculated for the reagent control, negative control, and each serum sample. All tests were run twice by different researchers in different periods.
Statistical analysis
All statistical procedures were performed by SPSS 15.0 software, Mann-Whitney U test was used in comparing the (medians) of two groups. p<0.05 was accepted for statistical significance. Articles were evaluated using a checklist derived from the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines [10].
Ethical statement
This study was approved by the DEU Local Ethics Committee (48/2012) and Three-Rs/3R principle was preserved without compromise throughout the study. The assessment was performed on animals in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences [8]. All the researchers of this study have the Certificate on Experimental Animal Use and the author in charge also holds FELESA C Category Certificate on Experimental Animal Use (Utrecht University, The Netherlands).
Results
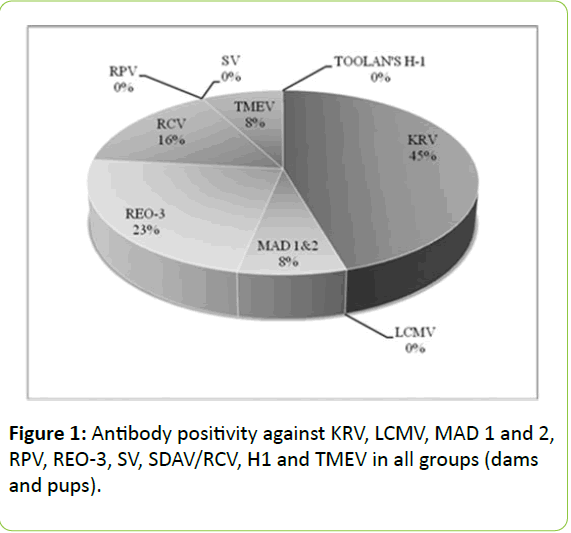
In our study, antibody response was not detected against LCMV, RPV, SV and Toolan's H1 virus neither in dams nor in pups of group 1 and group 2 (Figure 1). A high level of KRV antibody positivity (45%) attracts attention in all groups. High antibody levels detected in the young pups after delivery suggests intrauterine transmission of antibodies from the mothers; whereas these levels were observed to decrease after day 21 and eventually after day 60 they reach the level that only indicates previous infections. Just a similar situation is also notable for RCV.
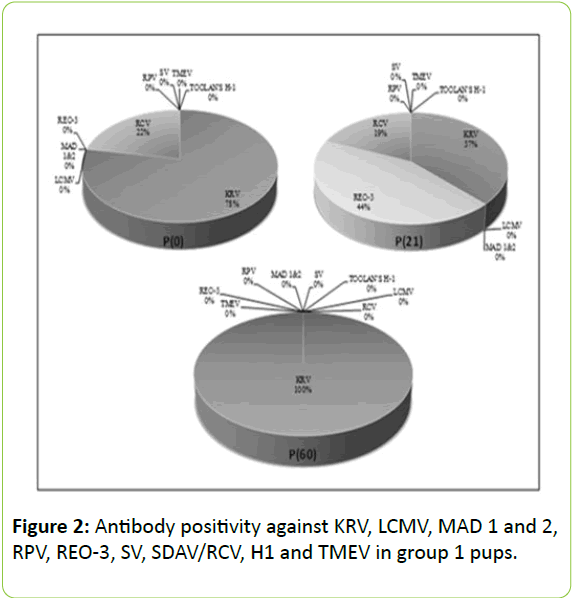
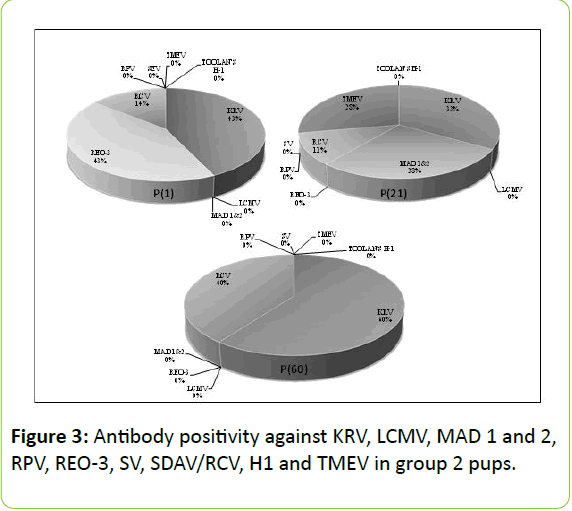
As for the agents other than MAD 1 and 2, REO-3 and TMEV, there was no statistically significant difference between fostered-pups and those left with their biological mothers (p>0.05). For REO-3 virus, it was important for the pups to stay with their biological mothers and nursed by them in first 24 hours (Figures 2 and 3).
Transmission of REO-3 virus antibodies from dams to pups was found significantly higher in Group 2 P(1) which were hosted for 24 hours with their biological mothers when compared with those in group 1 P(0) which were sacrificed immediately after birth (p=0.006). Antibody positivity against MAD 1 and 2 and TMEV infection was found only in the dams and pups of group 2 P(21) and not observed in the other groups (Figure 3). Serological profiles of all experimental groups are shown in Table 1.
| Groups | Features | Reason of formation |
|---|---|---|
| Group 1,P(0) | Dams and pups scarified just after birth | Transplacental antibody transmission |
| Group1, P(21) | Pups were housed with their biological mother and breastfed for 21 days after birth | Transplacental transmission and antibody transmission from biological mother via breastfeeding |
| Group 1, P(60) | Pups were housed with their biological mother and breastfed for 21 days after birth then fed ad libitum by standard pellet diet until day 60. | Transplacental transmission, antibody transmission from biological mother via breastfeeding, micro and macro environmental effects |
| Group 2,P(1) | Pups were housed with their biological mother for 24 hours after birth | Transplacental transmission and antibody transmission from biological mother via breastfeeding during 24 hours |
| Group 2, P(21) | Pups transferred to foster-dam just after birth and breastfed by foster-mother for 21 days | Transplacental transmission, antibody transmission from foster-damviabreasfeeding |
| Group 2, P(60) | Pups were housed and breastfed by foster-dam for 21 days then fed ad libitum by standard pellet diet until day 60 | Transplacental transmission, antibody transmission from foster-dam via breasfeeding, micro and macro environmental effects |
Table 1: Study groups and features.
Discussion
In recent years, a significant increase is apparent in the variety and quality of laboratory animals internationally, regarding the need in scientific research area. Microbiological monitoring is also improved along with the increased variety of genetically defined animal models. The researchers, pointing out the necessity for high security measures on this matter, indicate that we need reliable guide sources and programmed animal experiments for pathogen detection [11]. Identifying common pathogens and taking due preventive precautions constitute fundamental steps of microbiological monitoring implemented on laboratory animals. Although detecting high-risk pathogens is the priority of microbiological monitoring protocols, our main objective is tracking the incidence trends of infectious agents over years and questioning the sufficiency of the standard conditions in the colony [12]. Information on the microbiological status of the experimental animals (laboratory animal) and their environment will be the key to achieve repeatable and reliable access to scientific data by using the minimum number of animals (Reducement) and to improve animal welfare (Refinement).
Cross-fostering practice is commonly utilized in breeding of laboratory animals. This is an important practice for the survival of the pups whose dams died in lactation period or while giving birth to a bigger pup. Passive immune agents that protect pups against various infectious agents in the first days of life are acquired from their mothers through the placenta and colostrum [5,6]. Therefore staying with their mothers after birth and nursing is very important for the offspring. In our study, serological profiles against certain viral agents were compared between pups housed with their biological mothers or transferred to foster-mothers’ cage immediately after birth and the effects of foster-mother usage were evaluated. At this point, FELESA recommendations on microbiological monitoring of production colonies and research units were adhered and viral agents as well as study methods were determined accordingly [13]. All serological tests in this study were performed by ELISA method. ELISA is frequently preferred in routine microbiological monitoring of the laboratory rats and mice, for being easily applicable and objectively assessable. It has been indicated in some studies that the serum samples found to be positive by ELISA should be confirmed by IFAT. However, further IFAT confirmation could not be performed in this study because serum samples were not enough for further analysis after testing for antibodies against nine different viruses.
Maternal antibodies are known to protect pups against environmental pathogens. Transmission of antibodies generally occurs through placenta or lactation from infected or naturally immunized mothers to pups.6 In the study by Offit et al. [14] female mice were parenterally inoculated with non-infectious Rota virus (RRV) and observed that they developed specific antibodies over time. These authors demonstrated the presence of immune response against RRV-induced gastroenteritis in the mice born from these dams. This study showed that immune system factors are maternally transferred from such passively immunized dams to their pups.
Arthwohl et al. [15] however, investigated the effects of fostering in mice against Murine Norovirus, Helicobacter spp. and Murine Hepatitis Virus and the level of protection against these agents after two years. Fostered-pups showed no difference in the incidence of these infectious agents. Also in our study, the fostered-pups or those left with the their biological mothers displayed no statistically significant difference with respect to the agents other than MAD 1 and 2, REO-3 and TMEV (p>0.05). REO-3 virus is known to have direct contact transmission by direct contact but with extremely low contagiousness among laboratory rodents. Transmission from infected mothers or cage materials is reported to be limited [16]. In our study, REO-3 antibody positivity was found to be 23% as shown in the Figure 1, when all groups were assessed together. When individual sub-groups were assessed separately, antibody positivity against RE0-3 was detected in both birth- and fostermothers but not in any pups of group 1 P(0), group 1 P(60) and group 2 P(60). In the pups, antibody transition during pregnancy was not detected; furthermore REO-3 infection due to direct contact with environmental factors was not determined in later days of life, which supports that transition is rare and random. On the other hand, housing the pups and nursing by their biological mothers for the first 24 hours appears to be significant in terms of REO-3. Thus, pups can be protected during the first moments of life against this virus which is transmitted through various environmental factors, secretions and direct contact.
In our study, antibody positivity against MAD 1 and 2 and TMEV was determined in sera of dams and pups of group 2 P(21) only, but not in other groups (Figure 3). Both viral agents show transmission via body secretions, direct contact or intestinal tract. Determining antibody positivity only in this group suggested that foster-mothers acquired both viruses from a source and transferred to fostered-pups through nursing. Considering serum antibody levels of these fostered-pups which were housed with foster-mothers for 21 days after birth, it may be assumed/anticipated that serum may become negative over time. The fact that the antibody positivity against both viral agents was around 8% when all groups were assessed, it is supporting their low incidence among laboratory rats [17,18]. Based on these data, we can claim these viruses are seen very seldom in our facility; also this foster-mother might have acquired these viruses from the environment and transferred to pups through nursing.
In the literature, LCMV and Toolan's H1 virus infections in the laboratory rat colonies are reported to be rare [19]. LCMV, Toolan's H1 and RPV was not identified in a study conducted by the collaboration of various research centers, universities and biotechnology companies that bred and maintained laboratory mice and rats by conventional methods between 2004-2009 in Australia [20]. Antibodies were not detected in any dams or pups in our study too, against Toolan's H1 and LCMV, which is a viral zoonotic agent. Our study is consistent with the above mentioned study, which, in this respect, was one of the most comprehensive studies of the recent years.
Sendai virus is one of the most important infectious agents for laboratory rats and mice. Sendai virus is highly infectious and spreads via direct contact as well as inhalation [17,20]. It is a remarkable finding that no antibody positivity against SV and thereby no previous SV infection was determined in any of our study groups. This may indicate housing and hygiene conditions and care practices in our breeding and stock units are adequate enough at least against this agent which can be transmitted in a colony via inhalation.
Serum samples of 392 young rats were obtained from 20 different units in Argentina, engaged in conventional breeding of laboratory animals between 2006 and 2008. The incidence of Kilham Rat Virus (KRV) was reported to be 51.8% (203/392) in these sera. In the same period, KRV infection was not determined in the units engaged in SPF breeding as well [21]. The highest antibody positivity in our study was against KRV (45%) in all groups. High levels of antibody positivity determined in young pups after delivery suggested intrauterine transmission whereas these levels decreased from the 21st day on. Furthermore, antibody positivity related to previous infections was noted from the 60th day on. This proves that KRV was predominant in our breeding and stock colony and this viral infection may emerge in utero and from the first moments of life and immunize the rats. Appropriate disinfection particularly of base materials and cages should be considered important and due measures should be taken against this agent which can be transmitted by secretions such as urine, feces, vomit, and milk. Because, KRV was determined to be negative in conventionally bred rats in a five-year study carried out by McInnes et al. [18] in Australia, which is an indication that such measures would help. Zenner et al. [19] have also reported that the incidence of infectious agents in the colony decreased through regularly conducted monitoring and hygiene protocols.
Conclusion
It is obvious that routine microbiological monitoring is a significant requirement especially in the facilities engaged in conventional breeding and maintaining. Initially, this situation can be perceived as an unnecessary or futile work in terms of manpower and financial burden. However, it will improve the quality of the colonies and the work carried out with these animals, and also it will prevent risking the whole colony in case of any infection. On the other hand, we have concluded that foster-mother usage would protect pups against a variety of viral agents in later periods of life and hence this practice should be considered to continue whenever required.
Conflict of Interest
None declared.
Funding
This research was supported by Dokuz Eylul University Scientific Research Projects Coordination Unit, by 2012 KB.SAG. 101 tag number.
References
- Collins MJ, Parker JC(1972) Murine virus contamination of leukemia viruses and transplantable tumors. J Natl Cancer Inst 49: 1139-1143.
- Nicklas W, Kraft V, Meyer B(1993) Contamination of transplantable tumors, cell lines and monoclonal antibodies with rodent viruses. Lab AnimSci 43: 296-300.
- Beynen AC, Gartner K, Van Zutphen LFM(2001) Standardization of animal experimentation.In: Van Zutphen LFM, Baumans V, Beynen AC, (eds). Principles of Laboratory Animal Science. Revised edition. Elsevier, Amsterdamp. 103-110.
- Commission of the European Communities.Fourth report from the Commission to the Council and the European Parliament on the statistics on the number of animals used for experimental and other scientific purposes in the member states of the European Union.
- Havenaar R, Meijer JC, Morton DB, Hoitinga JR, Zwart P(2001) Biology and husbandry of laboratory animals. In: Van Zutphen LFM, Baumans V, Beynen AC, eds. Principles of Laboratory Animal Science. Revised edition. Elsevier, Amsterdamp. 19-75.
- Grindstaff JL, Brodie III ED, Ketterson ED(2003) Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proceedings Royal Society, London B 270: 2309-2319.
- Mähler-Convenor M, Berard M, Feinstein R, Gallagher A, Illgen-Wilcke B, et al.(2014) FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim 48: 178-192.
- National Research Council(1996) Guide for the Care and Use of Laboratory Animals, 7thedn. National Academy Press, Washington, DC.
- Close B, Banister K, Baumans V, Bernoth EM, Bromage N, et al.(1996) Recommendations for euthanasia of experimental animals: Part 1. DGXI of the European Commission. Lab Anim 30: 293-316.
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG(2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoSBiol 8:e1000412.
- Takanashi-Omoe H, Omoe K(2007) Animal experimentation in Japan: Regulatory processes and application for microbiological studies. Comp ImmunolMicrobiol Infect Dis 30: 225-246.
- Pritchett-Corning KR, Cosentino J, Clifford CB(2009) Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43: 165-173.
- Nicklas W, Baneux P, Boot R,Decelle T, Deeny AA, et al.(2002) FELASA(Federation of European Laboratory Animal Science Associations Working Group on Health Monitoring of Rodent and Rabbit Colonies). Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab Anim 36: 20-42.
- Offit PA, Dudzik KI(1989) Noninfectious rotavirus(strain RRV) induces an immune response in mice which protects against rotavirus challenge. J ClinMicrobiol 27: 885-888.
- Artwohl JE, Purcell JE, Fortman JD(2008) The Use of Cross-foster Rederivation to Eliminate Murine Norovirus, Helicobacterspp., and Murine Hepatitis Virus from a Mouse Colony. J Am Assoc Lab AnimSci 47: 19-24.
- Barthold SW, Smith AL, Bhatt PN(1993) Infectivity, disease patterns, and serologic profiles of reovirus serotypes 1, 2, and 3 in infant and weanling mice. Lab AnimSci 43: 425-430.
- National Research Council(1991) Infectious disease of mice and rats: a report of the Institute of Laboratory Animal Resources Committee on Infectious Disease of Mice and Rats. National Academy Press, Washington, D.C. USA.
- McInnes EF, Rasmussen L, Fung P, Auld AM, Alvarez L, et al.(2011) Prevalence of viral, bacterial and parasitological diseases in rats and mice used in research environments in Australia over a 5-y period, Lab AnimNY, USA. 11: 9-22.
- Zenner L, Regnault JP(2000) Ten-year long monitoring of laboratory mouse and rat colonies in French facilities: A retrospective study. Lab Anim 34: 76-83.
- Homberger FR, Thomann PE(1994) Transmission of murine viruses and mycoplasma in laboratory mouse colonies with respect to housing conditions. Lab Anim 28: 113-120.
- Cagliada MP, Carbone C, Ayala MA, Laborde JM, Maschi F, et al.(2010) Prevalence of antibodies against Kilham virus in experimental rat colonies of Argentina, Rev Argent Microbiol 42: 27-29.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences