Effect of supplementing essential oils on the in vitro methane production and digestibility of wheat straw
Hundal JS, Wadhwa M and Bakshi MPS
DOI10.21767/2572-5459.100014
Hundal JS, Wadhwa M and Bakshi MPS*
Department of Animal Nutrition, Guru Angad Dev Veterinary and Animal Science University, Ludhiana, India
- Corresponding Author:
- Bakshi MPS
Department of Animal Nutrition
Guru Angad Dev Veterinary and Animal Science University
Ludhiana-141004, India
Tel: 09569105009
E-mail: bakshimps@yahoo.com
Received Date: March 12, 2016; Accepted Date: April 09, 2016; Published Date: April 15, 2016
Citation: Hundal JS, Wadhwa M and Bakshi MPS. Effect of supplementing essential oils on the in vitro methane production and digestibility of wheat straw. J Anim Res Nutr 2016, 1:14. doi: 10.21767/2572-5459.100014
Copyright: © 2016 Hundal JS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The study was taken up to assess the effect of the pure essential oils (EOs) viz. cinnamaldehyde, carvacrol, carvone and limonene supplemented individually at 1 to 5% levels of the substrate DM (wheat straw) on the in vitro methane production and fiber degradation in a 4 × 7 factorial design. Supplementation of cinnamaldehyde and carvon, irrespective of their level had significantly (P < 0.01) higher net gas production (NGP), digestibility of neutral detergent fiber (NDFD) and true organic matter (TOMD), metabolizable energy (ME) availability and volatile fatty acids (VFAs) production from the substrate. The methane production was lowest (P < 0.01) in carvacrol followed by limonene and highest (P < 0.01) in carvone supplemented groups. Irrespective of the type of EO, the NGP and ME availability at 1% level of supplementation was comparable with control, while values of all other parameters were significantly (P < 0.01) lower than control and positive control. The NGP, NDFD and TOMD, ME availability, methane production and total and individual VFAs production was depressed significantly (P < 0.01) beyond 1% level of supplementation of EO. It was concluded that carvacrol or limonene supplementation beyond 1% level reduced the methane production but the digestibility of nutrients, volatile fatty acid production and ME availability from the substrate were also depressed significantly.
Keywords |
||||||||
| Essential oils; In vitro evaluation; Methane production; Nutrient digestibility | ||||||||
Introduction |
||||||||
| Mitigation of enteric methane emission and decreasing the carbon footprints of ruminants is one of the pressing challenges faced in the ruminant production sector. Not withstanding, because of the intricate relationships existing between the efficiency of feed fermentation in the rumen and methanogenesis, mitigation options have to be evaluated not just in terms of their effect on methane or total green house gases emissions but also on other rumen functional parameters and on their final consequences on animal production. | ||||||||
| The efficiency of energy and protein utilization in the rumen is relatively low and can be improved by the modulation of several metabolic pathways, including the inhibition of methane production and deamination in the rumen. This low efficiency not only reduces production performance, but also contributes to the release of pollutants to the environment [1]. The efficiency can be improved by modulating the activity of specific rumen microbial populations involved in the metabolic pathways. In recent years, the use of antibiotics as growth promoters in animal nutrition has been banned in the European Union because of its relation to the increase in the number of antibiotic-resistant bacteria to drugs which are used by humans. Consequently, new commercial additives are required that offer more safety, but can manipulate rumen fermentation. Thus, additives of vegetative origin, considered to be natural products, have been proposed to livestock producers as possible replacers of growth promoting antibiotics. The enteric methane production depends on dietary factors like soluble sugars, dietary lipids, level of feeding, roughage to concentrate ratio, type of forage, stage of maturity of forage, rate of passage of digesta, efficiency of feed conversion, processing and supplementation [2,3]. Besides polluting the environment, it also represents 2-10% loss in gross energy intake of feed. Because of negative correlation of methane production with energy utilization in ruminants, many efforts have been made to inhibit its production and to re-channel hydrogen to produce more propionate and microbial mass. It can be mitigated by the antimicrobial activity of natural plant extracts [4,5], feed additives [6] and phytogenics [7]. | ||||||||
| Fruit and vegetable wastes are good sources of different plant secondary metabolites like tannins, saponins and essential oils [8,9]. Cinnamaldehyde - a phenylpropanoid [(2E)-3-phenylprop-2-enal] is present in the bark of cinnamon trees and other species of the genus Cinnamomum. It is the main active component of cinnamon oil. Carvacrol is a monoterpenoid phenol [2-Methyl-5-(1-methylethyl)-phenol], with a characteristic pungent, warm odor and found in oregano (Origanum spp.). Limonene is a colorless liquid hydrocarbon classified as a cyclic terpene [1-Methyl-4-(1- methylethenyl)-cyclohexene] and is found in the citrus fruits. Carvone belongs to the family terpenoids [2-Methyl-5-(1- methylethenyl)-2-cyclohexenone] is most abundant in the oils from seeds of caraway (Carum carvi), spearmint (Mentha spicata), and dill. All these EOs have wide spectrum of antimicrobial activity against gram-positive and/or negative bacteria. Essential oils develop their action against bacteria through the interaction with the cell membrane. This interaction causes conformational changes in the membrane and results in the leakage of ions across the cell membrane and the loss of the trans-membrane ionic gradient. In most cases, bacterial growth is reduced and, in some cases, microbial death occurs [10]. This mechanism of action makes these essential oils more effective against gram positive bacteria, where the cell membrane can interact directly with hydrophobic compounds of essential oils [11]. The present in vitro study was conducted to screen the best, amongst the 4 EOs used, and the level/dose at what these should be supplemented to get best response with respect to enteric methane mitigation; with least effect on digestibility of nutrients using wheat straw as a substrate. | ||||||||
Material and Methods |
||||||||
| The EOs being aromatic in nature, are poorly soluble in water, and this causes many problems for studying their biological and pharmacological properties. Wadhwa et al. [12] revealed that amongst the different solvents like water, methanol, ethanol, petroleum ether and propylene glycol; EOs dissolved in methanol gave the best response with regards to in vitro NGP, digestibility of nutrients, methane and VFA production. Therefore, pure EOs viz. cinnamaldehyde, carvacrol, limonene and carvone were procured from Sigma- Aldrich chemicals and their 1 to 5% solutions were made with pure methanol. The antimicrobial properties of 4 essential oils at 1-5% of substrate DM was assessed by using wheat straw (WS) as the substrate. The wheat straw sample was finely ground to pass through 1mm screen of Willey mill. Since the essential oils were dissolved in pure methanol, therefore, besides using wheat straw as control; wheat straw plus methanol was used as positive control in this study. | ||||||||
| In vitro studies | ||||||||
| Three rumen fistulated rams were offered concentrate mixture (Maize 32, barley 20, soybean meal 15, groundnut extraction 15, rice bran 15, mineral mixture 2 and common salt 1% each) and green fodder in 50:50 ratio on DM basis. The rumen contents were collected before feeding at 0900 in a thermos flask flushed with CO2 and maintained at 39ÃÆââ¬Â¹Ãâà ¡C. The rumen contents were blended for 2-3 min in a blender and strained through four-layers of muslin cloth. The solution, containing 960 ml distilled water, 0.16 ml micro-mineral solution, 660 ml bicarbonate buffer, 330 ml macro-mineral solution and 1.6 ml resazurine (0.1%) were mixed in a Woulff flask (3 Litres capacity) with magnetic stirrer in a water bath at 39°C [13,14]. The mixture was continuously flushed with CO2. Then strained rumen liquor (SRL) was added to the buffer media in the ratio of 1:2. Essential oil dissolved in methanol; and methanol without essential oil were added to 100 ml calibrated glass syringes (Haberle Labortechnik, Germany) containing 375 ± 5 mg wheat straw with buffered rumen fluid. Syringes were incubated in triplicate in a water bath at 39ÃÆââ¬Â¹Ãâà ¡C and swirled every 60 min over a 24 h incubation period. If the volume of gas in the syringe exceeded 70 ml after 8 h the volume was recorded and the gas was expelled. After 24 h, the volume of gas produced in each syringe was recorded and the contents of syringes were transferred to spout-less beaker, boiled with neutral detergent solution for assessing the true OM and NDF digestibility. Each in vitro gas production set was repeated thrice in order to check any variation in the net gas production and other parameters. | ||||||||
| Methane estimation | ||||||||
| For CH4 estimation, 200 mg of substrate was incubated for 24 h with buffered rumen liquor and respective EO solution in duplicate. After the stipulated period, total gas production was measured. For CH4 estimation, representative gas was sampled from the headspace of syringe in an airtight syringe and injected into Netchrom 9100 gas chromatograph equipped with flame ionization detector (FID) and stainless steel column packed with Porapak-Q. The gas flow rates for N2, H2 and air were 15, 30 and 300 ml min-1, respectively. Temperature of injector oven, column oven and detector were 70, 50 and 100ÃÆââ¬Â¹Ãâà ¡C, respectively. A 50/50 mixture of CH4 and CO2 (Spancan; Spantech Products Ltd., England) was used as a standard. | ||||||||
| Estimation of volatile fatty acid | ||||||||
| After 24 h of incubation, a 5 ml aliquot of fluid from each syringe was mixed with 1 ml of 25% meta-phosphoric and kept for 1 h at ambient temperature [15]. Thereafter, it was centrifuged at 5500 rpm for 10 min and clear supernatant was collected and stored at -20ÃÆââ¬Â¹Ãâà ¡C until analyzed. The volatile fatty acids were estimated using Netchrom 9100 gas chromatograph equipped with glass column (packed with chromosorb 101) and flame ionization detector [16]. Temperature of injection port, column and detector was set at 250ÃÆââ¬Â¹Ãâà ¡C, 175ÃÆââ¬Â¹Ãâà ¡C and 270ÃÆââ¬Â¹Ãâà ¡C, respectively. The flow rate of carrier gas (N) through the column was 15 ml min-1; and the flow rate of H2 and air through FID was 30 and 300 ml min-1, respectively. Sample (2 μl) was injected through the injection port using a Hamilton syringe (10 μl). Individual VFA’s of the samples were identified on the basis of their retention time and their concentration (mmol) and calculated by comparing the retention time as well as the peak area of standards after deducting the corresponding blank values. | ||||||||
| Analytical methods | ||||||||
| The finely ground samples of the substrate were analyzed for dry matter (DM), crude protein (CP), ether extract (EE) and total ash [17] and neutral detergent fiber (NDF) [18]. For ammonia estimation, 5 ml of supernatant was mixed with 1N NaOH and steam distillated and the NH3 evolved was collected in boric acid solution containing mixed indicator and titrated against 0.01N H2SO4 [17]. | ||||||||
| Statistical analyses | ||||||||
| The data were analyzed by 4×7 factorial design [19], taking different EOs as one factor and levels of EOs as second factor. The data was analyzed by using SPSS [20] version 16.0 and the means were tested for the significant difference by using Tukey’s b test. | ||||||||
Results |
||||||||
| Effect of type of essential oils irrespective of their level | ||||||||
| The results revealed that irrespective of the level of EO supplementation, NGP varied (P < 0.01) from 54 (carvacrol) to 117 ml/g DM/24 h (carvone). The NGP, digestibility of NDF, true OM and ME availability from WS was similar in cinnamaldehyde and carvon supplemented groups, but significantly (P < 0.01) higher than that of carvacrol and limonene supplemented groups (Table 1). Cinnamaldehyde had an edge over carvone as far as these parameters were concerned. Carvacrol had the lowest (P < 0.01) digestibility of nutrients as compared to other supplemented groups. Carvacrol had the lowest methane production followed by limonene supplemented groups, but significantly (P < 0.01) lower than that of cinnamaldehyde and carvone supplemented groups, at the cost of impaired digestibility of nutrients. Supplementation of cinnamaldehyde and carvone resulted in lower NH3-N production as compared to carvacrol and limonene supplementation, but the differences were nonsignificant. | ||||||||
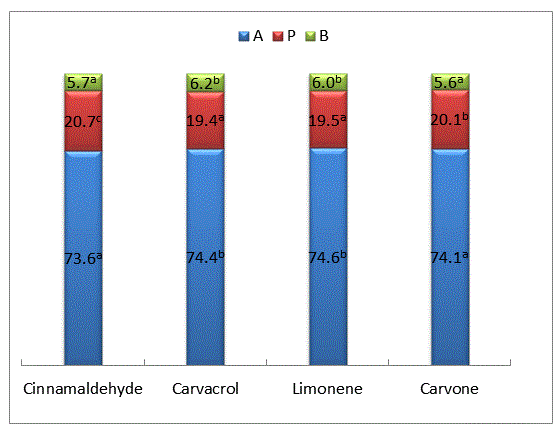
| Supplementation of cinnamaldehyde resulted in significantly higher production of total (P < 0.05) and individual VFAs (P < 0.01), carvone followed the trend (Table 2). This resulted in lower (P < 0.01) A: P ratio. The relative proportion of acetate decreased, while that of propionate increased (P < 0.01) when cinnamaldehyde or carvone was supplemented to the diet (Figure 1). The carvacrol and limonene supplementation to the substrate increased the relative proportion of butyrate significantly (P < 0.01). | ||||||||
| Effect of level of essential oils, irrespective of their type | ||||||||
| The NGP at 1% level of EOs supplementation was comparable to control (WS), but significantly higher (P < 0.01) than the positive control (WS+ methanol). Beyond 1% the NGP from substrate (wheat straw) decreased (P < 0.01) linearly with increase in level of EO supplementation, mainly due to linear decrease (P < 0.01) in the digestibility of NDF and that of true organic matter (TOM) as compared to both control as well as positive control (Table 3). The ME availability from the substrate followed the similar trend, except that beyond 2% there was no significant depression in ME availability. The methane production was comparable in control, positive control and upto 1% level of EO supplementation. As compared to control there was a significant (P < 0.01) depression in methane production, beyond 1% level of essential oil supplementation. The NH3-N was comparable in positive control and at all the levels of EO supplementation, but was depressed significantly in all the groups as compared to control group. | ||||||||
| The TVFAs and acetate production in positive control (WS + Methanol) was depressed significantly (P < 0.01) as compared to the control group, but propionate and butyrate production was similar in both the groups (Table 4). Amongst the EO supplemented groups, the TVFAs and individual VFAs production were highest at 1% level of supplementation, but significantly (P < 0.01) lower than control and positive control groups. Beyond 1% level of supplementation, these parameters decreased (P < 0.01) linearly, with increase in level of supplementation of essential oils. Reverse but significant (P < 0.01) trend was observed in case of acetate to propionate ratio, which increased (P < 0.01) with increase in level of supplementation of essential oils. The significant depression in the production of TVFAs and individual VFAs could be due to significantly (P < 0.01) reduced digestibility of substrate. Amongst the EO supplemented groups, the relative proportion of acetate increased (P < 0.01) linearly, while that of propionate decreased linearly, with the increase in level of EO supplementation. | ||||||||
Discussion |
||||||||
| Irrespective of the dose of EOs, supplementation of cinnamaldehyde had edge over other EOs as far as NGP, in vitro digestibilities, volatile fatty acid production and A: P ratio was concerned, but methane production was depressed significantly in carvacrol and limonene as compared to cinnamaldehyde and carvone. Earlier studies also revealed that peppermint [21,22], eucalyptus [23], thyme [24] cinnamon [25] and lemon [26] oils modified rumen microbial fermentation by decreasing ammonia N concentration through their impact on hyper-ammonia producing bacteria resulting in reduced deamination of amino acids, reduce methane emission, protozoa count and alter molar ratios of short chain fatty acids [27-30]. The anti-methanogenic effect of EOs in the rumen could be attributed to the presence of terpeniods and phenylpropanoids in oils. The EOs differ in chemical composition and have different effect on the same microbes. Different groups of rumen microbes also differ in sensitivity to the same EO. The EO containing a phenolic (e.g. carvacrol) or a carbonyl (e.g. cinnamaldehyde) compound demonstrated a stronger antimicrobial activity than EO that contains monoterpenes. Interactions among different EO and different EO components may affect their antimicrobial activity [31]. Arfa et al. [32] also reported that antimicrobial activity of carvacrol was related to its chemical structure and can be accumulated in the cell membrane. Its hydrogen-bonding ability and its proton-release ability may induce conformational modification of the membrane resulting in the cell death. Patra & Yu [33] reported that clove oil, eucalyptus oil, garlic oil, origanum oil, and peppermint oil significantly reduced methane production with increasing doses as compared with the control. However, apparent degradability of DM and NDF decreased linearly with increasing doses by all EOs except garlic oil. All the EOs decreased the abundance of archaea, protozoa, and major cellulolytic bacteria (Fibrobacter succinogenes, Ruminococcus flavefaciens, and R. albus) linearly with increasing EO doses. | ||||||||
| Irrespective of the EO supplemented, the NGP, digestibility of NDF and TOM, total and individual VFA and methane production were depressed linearly with the increase in dose of EOs. Similar trend in reduction in methane production with depression in the digestibility of nutrients was observed, when peppermint or clove oil extract were added to the substrate [21,34,35]. It is likely that the use of high doses of plant extracts and/or their secondary metabolites with antimicrobial activity decreased total microbial activity and diet fermentability [36]. Similar to ionophores like monensin (banned in EU since 2006), polyunsaturated fatty acids and essential oils act against gram positive bacteria, favouring propionate producing bacteria in the rumen, thereby encouraging alternative hydrogen sink to methane – propionate [37]. Linolenic acid, linoleic acid and Allium arenarium oil (garlic oil) reduced methane by 71%, 51% and 36% respectively, in vitro [38]. | ||||||||
| As compared to control, the addition of EOs depressed (P < 0.01) the ammonia-N concentration in the present study, confirming the earlier report [39]. Moreover, individual VFA proportions were affected by the dose of additives. The acetate to propionate ratio increased (P < 0.01) with increase in level of supplementation of essential oils. Improved A:P ratio was also observed on thymol supplementation [40]. Higher doses of cinnamon oil and cinnamaldehyde decreased total VFA and ammonia-N concentrations, although cinnamaldehyde had stronger effects compared with cinnamon oil [38,41]. | ||||||||
Conclusion |
||||||||
| It was concluded that carvacrol or limonene supplementation beyond 1% level reduced the methane production but the digestibility of nutrients, volatile fatty acid production and ME availability from the substrate were also depressed significantly. | ||||||||
Tables at a glance |
||||||||
|
||||||||
Figures at a glance |
||||||||
|
||||||||
References |
||||||||
|

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences