Use of a Probiotic to Ameliorate the Growth Rate and the Inflammation of Broiler Chickens Caused by Eimeria tenella Infection
Chih-Yuan Chen, Li-Tsen Chuang, Yue-Cheng Chiang, Chun-Li Lin, Yi-Yang Lien and Hau-Yang Tsen
DOI10.21767/2572-5459.100010
Chih-Yuan Chen1, Li-Tsen Chuang1, Yue-Cheng Chiang1, Chun-Li Lin1, Yi-Yang Lien2 and Hau-Yang Tsen1*
1Department of Food Science and Technology, Hung-Kuang University, Shalu, Taichung 43302, Taiwan, R.O.C
1Department of Veterinary Medicine, National Pingtung University of Science and Technology, Pingtung 91201, Taiwan, R.O.C
- Corresponding Author:
- Hau-Yang Tsen
Department of Food Science and Technology
Hung-Kuang University, No. 34, Chung-Chi Rd
Taichung 43302, Taiwan, R.O.C
Tel: 886-4-26318652
Fax: 886-4-26527731
E-mail: hytsen@sunrise.hk.edu.tw
Received date: December 14, 2015, Accepted date: January 14, 2016, Published date: January 21, 2016
Citation: Chen CY , Chuang LT , Chiang YC, et al. Use of a Probiotic to Ameliorate the Growth Rate and the Inflammation of Broiler Chickens Caused by Eimeria tenella Infection. J Anim Res Nutr 2016, 1:10. doi: 10.21767/2572-5459.100010
Copyright: © 2016 Chen CY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
A multistrain formula (MF) consisted of four selected lactic acid bacteria (LAB) strains was evaluated for its function to ameliorate the growth rate and inflammation of broiler chickens caused by Eimeria tenella parasitizing. Thirty one day broiler chickens were divided into 3 groups with 10 chickens in 2 cages per group. These groups were control, infected and MF groups. In control group, chickens were fed with 0.2 ml PBS and not challenged with E. tenella. In MF group, chickens were gavage fed with 0.2 ml of MF (109 CFU/ml) from day 1 to day 23. On day 14, each chicken in both the infected and MF groups was challenged with 5,000 sporulated E. tenella oocysts. Seven days post infection, it was found that the average body weight gain (ABWG) for chickens in the infected and MF groups were 281.51 ± 61.00 and 295.52 ± 62.16 g per chicken, respectively. Chickens in each group were then sacrificed and their cecal tonsils collected. It was observed that feeding MF could reduce the cecal lesion scores (LS) of the Eimeria infected chickens significantly. In addition, for chickens in the MF group, the levels of cecal gene expression of proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, and interferon (IFN)-γ, were found significantly lower, while anti-inflammation cytokine, ie, IL-10, was significantly higher than those of the chickens in the infected group. This study thus suggests that the MF, which previously has been shown to have antagonistic effect against Salmonella infection and the induced inflammation either in live or in heat killed form, may also be useful for the amelioration of the growth rate and the inflammation of broiler chickens caused by E. tenella infection.
Keywords
Probiotic; Chickens; Eimeria; Infection; Inflammation
Introduction
Eimeria infection of chickens may lead to increased mortality, malabsorption, inefficient feed utilization, impaired growth rate of the chickens and reduced egg production by layers [1]. Eimeria species, including Eimeria acervulina, brunetti, maxima, mitis, necatrix, praecox and tenella, are host specific and able to cause coccidiosis in chickens that lead to economic loss to poultry industry [2]. Each Eimeria species may have its specific inhabit location within the intestine of the host. For E. tenella, it is one of the most pathogenic Eimeria organisms that infect chicken ceca and parasitizes growing chickens [3].
Studies have demonstrated a dominant role for T-cells in immune response to Eimeria [4,5]. When E. tenella infected chicken, macrophages massively infiltrate into the chicken cecum at post-infection and secrete large amounts of cytokines. In general, cytokines, such as IL-12, IL-1β, IFN-γ, IL-6 and TNF-α are associated with inflammatory responses, while other cytokine, such as IL-10, favors the development of humoral mediated immunity and is implicated in antiinflammatory responses [5,6].
The use of probiotics in poultry is based on their functions to improve the gut flora and to enhance the immunomodulatory activity as well as the growth rate of host animals. For chickens, these functions involve the resistance to enteric infections, such as the protection against Salmonella infection [7,8] or Eimeria parasitizing [4,9-11]. Studies also have shown that for disease prevention and immune enhancement, multistrain probiotics are more effective than monostrain probiotics due to the additive and synergistic effects. For example, a umbination of the four LAB strains used in this study showed higher immunomodulatory activity than those of their monostrains [7,12]. In this study, we thus attempted to use the multistrain formula (MF) consisted of four lactic acid bacteria strains and to evaluate its protective function against coccidiosis in chickens. These four strains were selected by immunomodulatory activity using mouse macrophage 264.7 cells [13] and the combination of these four strains, either in live or heat killed form, has been shown to have protective effect against Salmonella infection and the induced cecal inflammation of chickens or mice [7,8]. In this manner, our objective is to develop a feed supplement which is able to reduce not only the Salmonella infection but also the Eimeria parasitizing of chickens, so that to ameliorate the growth rate and induced the inflammation of chickens infected by either of these pathogens.
Materials and Methods
Bacteria strains and preparation of multistrain formula (MF)
Four LAB strains, including Lactobacillus acidophilus (LASW), L. fermentum (LF33), L. plantarum (LPL05) and Enterococcus faecium (TM39), were equally combined into a multistrain formula termed as MF. The four strains used in MF were those with the basic probiotic properties, such as gastric juices tolerance, bile tolerance and the ability to adhere the cultured human intestinal epithelium cell line Caco-2 and chicken crop epithelial cells. In addition, these strains were those selected by immunomodulatory activity using mouse macrophage 264.7 cells [7,13]. The stock culture of each of these strains was maintained at -80°C in 20% glycerol. Before use, bacterial cells were propagated twice in lactobacilli MRS broth (Difco) containing 0.05% L-cysteine, each time for 24 hr at 37°C. Whole cells of each strain were obtained by centrifugation at 7,000 g for 10 min and washed twice and then suspended in sterilized PBS (pH 7.2). The cell counts of each LAB strains in the suspension were than determined using MRS agar plates. Prior to the feeding step, the MF was prepared by mixing 109 CFU/ml each of these LAB strains.
Parasites
The wild type strain of E. tenella [3] was obtained from the Department of Veterinary Medicine, National Pingtung University of Science and Technology, Pingtung, Taiwan. Oocystes were cleaned by floatation on 2.5% Postassium dichromate, washed three times with PBS, and enumerated using a hemocytometer prior to infection.
Experimental design and growth rate measurement
Newly hatched Arbor Acres broiler chickens were used in this study. Chickens were housed in Eimeria -free battery cages in the animal room of Hung-Kuang University (Taichung, Taiwan) and provided with regular feed and water ad libitum. Regular feed for broiler chicks was purchased from the Formosa Oil Seed Processing Co. Ltd. Taichung, Taiwan. The feed was regular broiler diet for chicks with age of 0-21 days purchased from Formosa oil seed processing Co. LTD (Taichung, Taiwan), which was free from antibiotic and consisted of fish meal, vitamin mixture, bioplex, and soy bean as well as corn meal (Table 1).
| Ingredient | g/kg |
| Corn meal | 565.9 |
| Soybean meal CP44% | 246.6 |
| Whole fat soybean meal | 83.2 |
| Fish meal CP65% | 45.3 |
| Soybean oil | 30.5 |
| Lime powder | 9.3 |
| Ca(H2PO4)2 | 10.9 |
| NaCl | 2.9 |
| Choline chloride | 0.9 |
| Vitamin prenix1 | 0.9 |
| Mineral premix2 | 0.9 |
| DL-Mathionine | 2.7 |
| Nutrition ingredient | Kcal/kg |
| Metabolic energy | 3056 |
| Crude protein% | 22.3 |
| Ca% | 0.91 |
| P% | 0.46 |
| Lysine% | 1.19 |
| Methionine% | 0.63 |
| Methionine + Cysteine% | 0.97 |
2Unit per kg feed: Co. 0.225 mg; Cu, 10.8 mg; Fe, 90 mg; Zn, 68.4 mg; Mn, 90 mg; Se, 0.18 mg.
Table 1: The feed formala for broiler chick (0-21 days age).
The experimental design was modified from that reported by Lee et al. [11]. Essentially, three feeding conditions were used. In the initial day, 30 of one day old chicks were randomly divided into 3 groups (10 chickens per group, 5 in each cage). They were the infected group, in which each chicken was fed with 0.2 ml PBS and challenged with E. tenella; the MF group, in which each chicken fed with MF (0.2 ml) was challenged with E. tenella; and the control group, in which chickens were only fed with 0.2 ml PBS without MF. Each chicken in the MF group was oral fed by gavages with MF (0.2 mL per chicken, 109 CFUmL-1) per day through initial day to the end of this study. On day 14, chickens in the infected and MF groups were oral challenged with 5000 sporulated E. tenella oocysts per chicken. Such dose was equivalent that used by Lee et al. [11]. The oocysts obtained were originally in 2.5% potassium dichromate for which, the oocysts were than collected by centrifugation at 2000 rpm (eppendorf centrifugator), washed, and pelleted by spinning down again. The oosysts in PBS suspension were then counted on Fuchs Rosenthal plate using microscope.
Through the experiment, the survival rate (%S) of the chickens was estimated by dividing the number of surviving chickens by the number of initial chickens. In addition, the body weights of the chickens in each group were measured each day, and relative weight gain (RWG), as well as body weight gain (BWG) were calculated. Relative weight gain (RWG); (RWG=BWG (per bird) × 100/BWG (control bird)) of the chickens in each group were determined by weighing the chickens from the day chickens challenged with Eimeria to the day chickens sacrificed, i.e. day 14 to day 21.
Seven days post infection, chickens in each group were euthanized with Forane® (Aesica Queenborough Ltd, UK) and sacrificed, and the cecal tonsils were collected to evaluate the lesion and the inflammation due to Eimeria infection [5,6]. The cecal mean lesion score (LS) as well as the expression levels of proinflammatory and anti-inflammatory cytokines in cecal tonsils were measured. The cecal lesion score (LS), was determined on a scale of 0 (none) to 4 (high) in a blinded fashion by three independent observers according to Johnson and Reid [14]. Each score 0-4 covers a range of gross lesions. Generally a score of 0=gross lesion absent; 1=a few scattered lesions; 2=a greater number of discrete lesions involving more of the affected zone of the intestine; 3=lesions extensively developed with coalescence and some thickening of the intestinal; and 4=extensive coalescence of lesions with thickening of the intestinal wall and bloody intestinal contents.
A microscope was used to examine scrapings for coccidia whenever there was doubt about the cause of a lesion.
Total RNA extraction and reverse transcription reaction
Immediate after the sacrifice of chickens, the cecal tonsils were collected, stored at -80°C until RNA extraction. Extraction of total RNA from cecal tonsil tissue and cDNA preparation were according to the procedures described by Chen et al. [7]. Total RNA was extracted from cecal tonsil tissue samples using PureLink RNA Mini Kit (Invitrogen) according to the manufacturer s instruction. To remove the possible contaminated DNA in RNA preparations, DNase I (deoxyribonuclease I) was used. The resultant RNA concentration was measured using a spectrophotometer at a 260-nm wavelength.
The cDNA synthesis was performed with 1 μg of total RNA using a reverse-transcription kit (SuperScript First-Strand Synthesis System, Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer s instruction. The primer used in this procedure was Oligo (dT) 12–18. The cDNA were stored at −20°C until real-time PCR assay.
Real-time quantitative PCR
Real-time PCR was used to quantify the expression level of the internal standard glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and cytokine genes from cDNA samples. The PCR reactions were prepared in a total volume of 20 μL containing 10 μL of KAPA FAST SYBR green I master mix (KAPA Biosystem, Woburn, MA), 1 μL of the cDNA, and 0.25 mM of each primer.
The sequence and other features of specific primers for chicken IL-1β, IL-6, IFN-γ, and IL-10 are shown in Table 2. Amplification and detection of specific products were performed using ABI 7500 system (Applied Biosystems) with the following temperature-time profile: one cycle of 95°C for 3 min, and 40 cycles of 95°C for 30 s, 60°C for 30 s, followed by 72°C for 40 s. To check the specificities of amplified products, the dissociation-curve mode was used (one cycle at 95°C for 10 s, 60°C for 15 s, and 95°C for 10 s) subsequent to amplification. The real-time quantitative PCR data were analyzed using the 2−ΔΔCt method of Livak and Schmittgen [15]. Briefly, the relative level of each mRNA normalized to the GAPDH gene was calculated using the following equation: fold change=2Ct target (control) – Ct target (treatment)/2Ct GAPDH (control) − Ct GAPDH (treatment).
| Type1 | Target cDNA2 | primer | Sequences (5 -3 ) | GenBank accession no. | References |
|---|---|---|---|---|---|
| Reference | GAPDH | Forward | GTCAGCAATGCATCGTGCA | K01458 | Berndt et al., 2007 |
| Reverse | GGCATGGACAGTGGTCATAAGA | ||||
| Proinflanmmatoty | IL-1β | Forward | GCTCTACATGTCGTGTGTGATGAG | AJ245728 | Kaiser et al., [6] |
| Reverse | TGTCGATGTCCCGCATGA | ||||
| IL-6 | Forward | GCTCGCCGGCTTCGA | AJ250838 | Kaiser et al., [6] | |
| Reverse | GGTAGGTCTGAAAGGCGAACAG | ||||
| Th-13 | IFN-γ | Forward | GCCGCACATCAAACACATATCT | NM_205149 | Berndt et al., 2007 |
| Reverse | TGAGACTGGCTCCTTTTCCTT | ||||
| Th-23 | IL-10 | Forward | CATGCTGCTGGGCCTGAA | AJ621614 | Kaiser et al., [6] |
| Reverse | CGTCTCCTTGATCTGCTTGATG | ||||
| 1The Experimental Conditions Were As Those Described In Material And Methods. 2GAPDH: Glyceraldehyde 3-Phosphate Dehydrogenase; IFN: Interferon; IL: Interleukin. 3Th: T Helper Cell. |
|||||
Table 2: Primers and sequences for real-time PCR1.
Statistical analysis
Average body weight gain (ABWG) and mean lesion scores (LS) were subjected to one-way analysis of variance using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL). Mean values of treatment groups were compared using the Duncan s multiple range test and differences were considered statistically significant at P<0.01 and P<0.05.
Result
Body weight gain
Since E. tenella is one of the most pathogenic Eimeria organisms that infect chicken cesa and parasitize growing chicks, in this study for chicken in the infected and MF group, only 5000 sporulated E. tenella oocycts were oral fed to each chicken. This dose of infection was that used by Lee et al. [11]. From day 14 to day 21 of the feeding period, i.e. the day of Eimeria challenge and the day of chicken sacrifice, we found that the average body weight gain (ABWG) of infected chickens and MF treated chickens were 281.51 ± 61.00 and 295.52 ± 16 g per chicken, respectively. The latter was close to that of the control group. Despite the high standard deviation of ABWG of chickens in Table 3, when% of RWG were measured from day 14 to day 21, the RWG estimated for chickens in the infected group and control group were 93.83% and 100%, respectively. Thus, Eimeria infection would reduce the growth rate of chickens. However, MF feeding seems to be able to improve the RWG of the infected chickens, i.e. from 93.83% to 98.30%, which was close to that of the control group (Table 3).
| Groups | %S1 | Body weight/chicken before infection (g)2 | Body weight/chicken after 7 days post infection (g)2 | ABWG (g/chicken)2 | %RWG |
|---|---|---|---|---|---|
| Control group | 100 | 354.64 ± 29.85a | 654.64 ± 52.15a | 300.01 ± 40.99a | 100 |
| Infected group | 100 | 357.09 ± 29.19a | 638.60 ± 62.44a | 281.51 ± 61.00a | 93.83 |
| MF + infected group | 100 | 343.70 ± 30.36a | 639.20 ± 57.05a | 295.52 ± 62.16a | 98.5 |
2Average body weights of 10 chickens were counted from day 14, the day before the Eimeria challenge, to day 21, the day 5 of the 10 chickens scarified. ABWG means the average weight gain.
Table 3: Effects of the MF against E. tenella infection of chickens based on different parameters.
Protective effects of MF against E. tenella infection
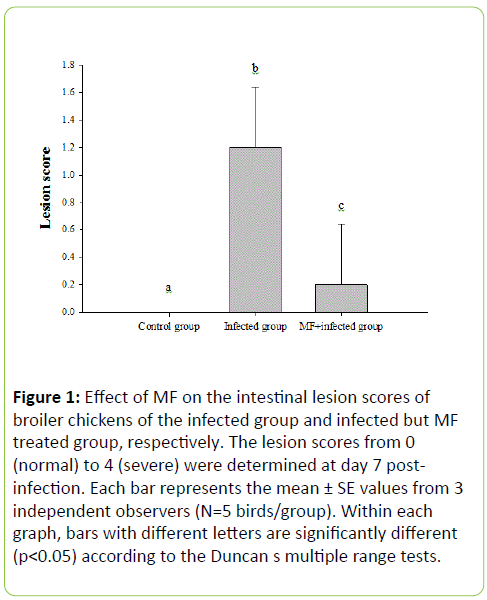
In any of the three groups, no chicken died after challenge with E. tenella coccidial. Seven days post-infection, chickens in each group were sacrificed and the cecal mean lesion scores (LS) were measured. For chickens in the infected, MF and control group, the mean LS scores were determined as 1.2 ± 0.44, 0.2 ± 0.44, and 0, respectively (Figure 1). Perhaps due to the low dose of Eimeria infection, the LS for chickens in the infected group was only 1.2 ± 0.44, feeding MF could significantly reduce the LS of the infected group to 0.2 ± 0.44, which was close to that of the control group, i.e. 0.
Figure 1: Effect of MF on the intestinal lesion scores of broiler chickens of the infected group and infected but MF treated group, respectively. The lesion scores from 0 (normal) to 4 (severe) were determined at day 7 postinfection. Each bar represents the mean ± SE values from 3 independent observers (N=5 birds/group). Within each graph, bars with different letters are significantly different (p<0.05) according to the Duncan s multiple rnge tests.
Comparison of the gene expression levels for cytokines in chicken cecum
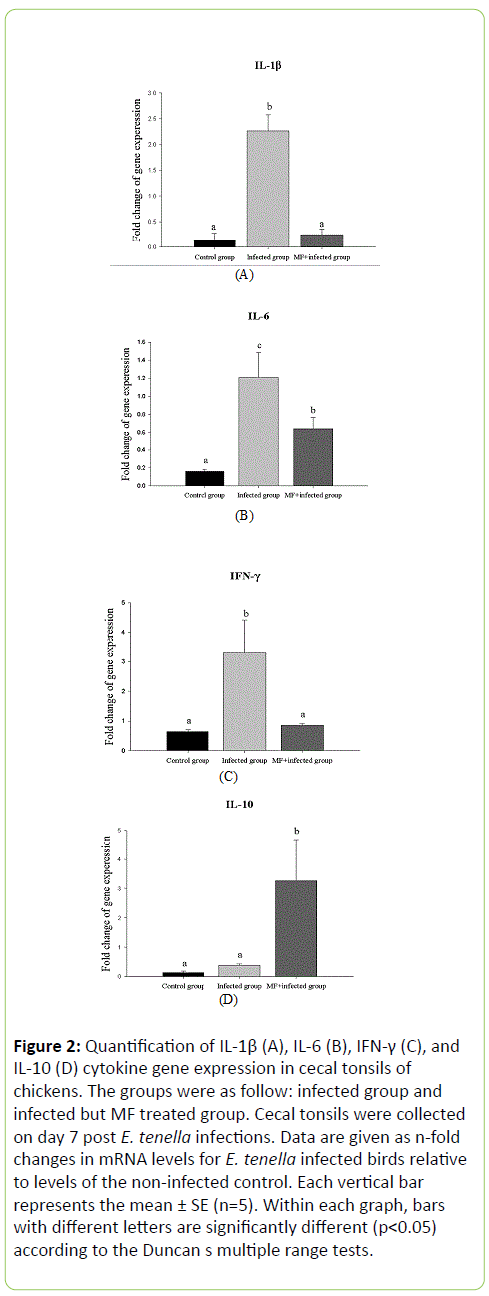
The levels of pro-inflammatory cytokines, i.e. IL-1β, IL-6 and IFN-γ, in the chicken cecal tonsils were measured. Results showed that 7 days post infection, the expression levels of IL-1β, IL-6 and IFN-γ in the chickens of the infected group were significantly higher than those of the MF treated, and the control groups (Figure 2A-2C). On the other hand, the expression level of anti-inflammatory cytokine, ie, IL-10, was significantly enhanced in chickens of the MF group (Figure 2D). Thus, oral feeding chickens with MF was able to reduce the inflammation and enhance the anti-inflammation activity of the E. tenella infected chickens.
Figure 2: Quantification of IL-1β (A), IL-6 (B), IFN-γ (C), and IL-10 (D) cytokine gene expression in cecal tonsils of chickens. The groups were as follow: infected group and infected but MF treated group. Cecal tonsils were collected on day 7 post E. tenella infections. Data are given as n-fold changes in mRNA levels for E. tenella infected birds relative to levels of the non-infected control. Each vertical bar represents the mean ± SE (n=5). Within each graph, bars with different letters are significantly different (p<0.05) according to the Duncan s multiple range tests.
Discussion
With an attempt to develop a feed supplement able to reduce not only the Salmonella infection but also the Eirmeria parasitizing of chickens, in this study, we evaluated the effect of a multistrain formula (MF) to reduce the coccidiosis and the induced cecal inflammation of chickens challenged with E. tenella. This MF, either in its live form or heat killed form, has been shown to have immunomodulatory activity and protective effect against Salmonella infection and the induced cecal inflammation of broiler chickens or mice [7,8]. As described earlier, the experimental method of animal study was essentially according to that reported by Lee et al. [11], except that in their study, 70 day-old broiler chicks randomly assigned to 7 pens (n=10/pen) were used to evaluate the protective effects of feeding Mito Grow, a pediococcus-based probiotic, on coccidiosis in broiler chickens. Also, in their study, only three feeding conditions were used. Their feeding groups of chickens were regular diet group, infected group (regular diet and Eimeria infected), and infected plus probiotic group. In addition, for the infected group, each chicken was challenged with 5000 oocysts.
Through the whole study, none of the chickens in any of our experimental groups, particularly in the Eimeria infected group, died, and the cecal lesion scores were low, i.e. 1.2 ± 0.44. Such result could be due to the low dose of E. tenella oocysts challenged per chicken. However, even though, significant difference in the cecal LS between chickens in the infection group and control group was observed (Figure 1). Similar results have been observed by Song et al. [16]. Since it was reported that for chickens orally inoculated with sporulated oocysts of E. tenella, 6-7 days post challenge would be an optimum time for chicken sacrifice and cecal lesion score evaluation [17,18], in this study, chickens were sacrificed on the 7th day post infection and the cecal lesion scores as well as the cytokine level in cecal tonsil were determined. Results showed that the lesion scores of chickens raised by Eimeria infection, ie. 1.2 ± 0.44, could be reduced by MF treatment, ie. to 0.2 ± 0.44.
As for the effect of probiotics on the chicken growth, it is desirable to see the improving of the weight gain for mice fed with probiotic supplement. Dalloul et al. [9] reported that treatment with a Lactobacillus-based probiotic could significantly reduce the fecal oocyst in broiler chickens infected with E. acervulina and increase the weight gain. For broiler chickens in the infected group and MF groups, we found that the ABWG were 281.51 ± 61.00 and 295.52 ± 62.16 g per chicken, respectively. Although the standard deviation of the ABWG for chickens is there two groups was high, the multistrain probiotic seems to be able to enhance the growth rate of the infected chickens.
To understand the interaction between Eimeria infection and chicken immune response, and the role(s) of parasite surface antigens in mediating chicken innate or adaptive immune responses, including the determination of mRNA transcription levels of chicken cytokines, such as IL-1β, IFN-γ, IL-6 and IL-10, several studies have been conducted [19,20].
For example, it has been reported that expression of the proinflammatory and Th1 cytokines, such as IFN-γ, IL-1β, IL-6, IL-12, IL-15, IL-17, and IL-18, fold increase in the chicken intestinal intraepithelial lymphocytes when measured different days post E. maxima infection [19]. These reports showed that for chickens inoculated with 1 × 104 sporulated oocysts of E. maxima, the proinflammatory cytokines, such as IFN-γ, IL-12 and IL-10, during the time 3-4 days and 6-7 days post infection, while IL-1β, during 4 to 6 days post infection, their gene expression levels were fold increased. As mentioned earlier, considering the time for cecal LS evaluation and for cytokine gene expression, in this study, chickens in each group were sacrificed on the 7th day post infection and the cecal tonsils were collected for the assay of cytokines expression. Under such conditions, results showed that significant difference in the expression levels of IL-1β, IL-6, IFN-γ and IL-10 were observed between the chickens in the infected and the MF groups (Figure 2).
Over expression of the proinflammatory cytokine can be detrimental to the host. For cecal coccidiosis, the upregulation of IL-1β in the chicken cecum was measured when chickens were infected with E. tenella [17]. Also, IL-6 is produced during immune response to parasite infection [6]. Interferon-γ is a key cytokine orchestrating the development of cellular mediated immunity and its expression has been shown to be regulated by the induction of IL-10 [20]. Eimeria infection may evoke high IFN-γ level in chickens at least 3 weeks of age. Our results showed that for E. tenella infected chickens, the expression levels of proinflammatory cytokines, such as IL-1β, IL-6 and IFN-γ, were induced on the 7th day post infections, and such expression level could be reduced significantly with MF treatment (Figure 2A-2C). On the other hand, IL-10, which may play a crucial role in changing the Th bias during infection with Eimeria [21], enhanced in the MF treated group as compared with that of the chickens in the infected group (Figure 2D).
Judged from the LS, the %RWG and the expression levels of cytokines of chickens in the control, infected and MF groups, the MF probiotic, in addition to its antagonistic effect against Salmonella infection [7], may also be useful as an effective probiotic to protect chickens against the Eimeria infection. Moreever, since this MF product, even after heat killing, preserves its immunomodulatory activity and autagonistic effect against Salmonella infection of chickens and mice [7,8], thus it is possible that the MF used in this study, even after the heat processing step during feed procession may also preserve its protective function against Eimeria infection of chickens. Finally, it should be reminded that prior to this industrial use, further studies including the use of different dosages of viable or heat killed MF to feed chickens challenged with different dosages of Eimeria spp., should be performed. In addition, the cost of industrial production of such probiotic and also the optimal quantity for chicken feeding should be estimated. Also, a field trial may be required prior to the large scale industrial use of this probiotic product.
Acknowledgement
We would like to thank the National Science Council (NSC), Taipei, Taiwan for supporting this work. The project No for NSC is NSC 101-2324-B-241-001.
References
- Lillehoj HS, Min W, Dalloul RA (2004) Recent progress on the cytokine regulation of intestinal immune responsed to Eimeria. Poultry Science 83: 611-623.
- Lillehoj HS, Lillehoj EP (2000) Avian coccidiosis. A review of acquired intestinal immunity and vaccination strategies. Avian Diseases 44: 408-425.
- Shirley MW, Smith AL, TomLey FM (2005) The Biology of Avian Eimeria with an emphasis on their control by vaccination. Advances in Parasitology 60: 285-330.
- Dalloul RA, Lillehoj HS (2005) Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Diseases 49: 1-8.
- Dalloul RA, Lillehoj HS (2006) Poultry coccidiosis: Recent advancements in control measures and vaccine development. Expert Review of Vaccines 5: 143-163.
- Lynagh GR, Bailey M, Kaiser P (2000) Interleukin-6 is produced during both murine and avian Eimeria infections. Veterinary Immunology and Immunopathology 76: 89-102.
- Chen CY, Tsen HY, Lin CL, Yu B, Chen CS, et al. (2012) Oral administration of combination of select lactic acid bacteria strains to reduce the Salmonella and inflammation of broiler chickens. Poultry Science 91: 2139-2147.
- Chen CY, Tsen HY, Lin CL, Lin CK, Chuang LT, et al.( 2013) Enhancement of the immune response against Salmonella infection of mice by heat-killed multispecies combinations of lactic acid bacteria. Journal of Medical Microbiology 62: 1657-1664.
- Dalloul RA, Lillehoj HS, Shellem TA, Doerr JA (2003) Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poultry Science 82: 62-66.
- Tierney J, Gowing H, Van Sinderen D, Flynn S, Stanley L, et al. (2004) and Mulcahy G. In vitro inhibition of Eimeria tenella invasion by indigenous chicken Lactobacillus species. Veterinary Parasitology 122: 171-182.
- Lee SH, Lillehoj HS, Dalloul RA, Park DW, Hong YH, et al. (2007) Influence of Pediococcus-based probiotic on coccidiosis in broiler chickens. Poultry Sciece 86: 63-66.
- Timmerman HM, Koning CJ, Mulder L, Rombouts FM, Beynen AC, et al. (2004) Monostrain, multistrain and multispecies probiotics-A comparison of functionality and efficacy. International Journal of Food Microbiology 96: 219-233.
- Tsai CC, Liang HW, Yu B, Hsieh CC, Hwang CF, et al. (2011) The relative efficacy of different strain combinations of lactic acid bacteria in the reduction of populations of Salmonella enterica Typhimurium in the livers and spleens of mice. FEMS Immunology and Medical Microbiology 63: 44-53.
- Johnson J, Reid WM (1970) Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Experimental Parasitology 28: 30-36.
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402-408.
- Song X, Xu L, Yan R, Huang X, Shah MA, et al. (2009) The optimal immunization procedure of DNA vaccine pcDNA-TA4-IL-2 of Eimeria tenella and its cross immunity to Eimeria necatrix and Eimeria acervulina. Veterinary Parasitology 159: 30-36.
- Laurent F, Mancassola R, Lacroix S, Menezes R, Naciri M, et al. (2001) Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infection and Immunity 69: 2527-2534.
- Shah MA, Song X, Xu L, Yan R, Song H, et al. (2010) The DNA-induced protective immunity with chicken interferon gamma against poultry coccidiosis. Parasitology Research 107: 747-750.
- Hong YH, Lillehoj HS, Lillehoj EP, Lee SH (2006) Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Veterinary Immunology and Immunopathology 114: 259-272.
- Chow YP, Wan KL, Blake DP, Tomley F, Nathan S, et al. (2011) Immunogenic Eimeria tenella glycosylphosphatidylinositol-anchored surface antigens (SAGs) induce inflammatory responses in avian macrophages. PLoS one 6: e25233.
- O’Garra A, Murph KM (2009) From IL-10 to IL-12: how pathogens and their products stimulate APCs to induce TH1 development. Nature Immunology 10: 929-932.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences