Milk Fat Depression: Etiology, Theories, and Soluble Carbohydrate Interactions
Koch LE, Lascano GJ
DOI10.21767/2572-5459.100046
Department of Animal and Veterinary Sciences, Clemson University, Clemson, SC 29634, USA
- *Corresponding Author:
- Lascano GJ
Department of Animal and Veterinary Sciences
Clemson University, Clemson
SC 29634, USA
Tel: (864) 656-1745
E-mail: glascan@clemson.edu
Received date: March 03, 2018; Accepted date: May 17, 2018; Published date: May 30, 2018
Citation: Koch LE and Lascano GJ (2018) Milk Fat Depression: Etiology, Theories, and Soluble Carbohydrates. J Anim Res Nutr 3: 2: 2.
Copyright: © 2018 Koch LE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Milk fat depression is a metabolic disorder characterized by a sustained reduction in milk fat and has challenged producers and scientists for over a century. Nutritionists have incorporated supplemental fat in dairy rations for years as a means to increase the energy density of the diet, but often decreased fiber digestibility or milk fat depression was observed. The rumen microbial populations were thought to play a major part in why producers had difficulty using high levels of unsaturated fat. Many different theories evolved throughout the century into the causes of milk fat depression that often appears ex nihilo. Such theories involved concepts centered on substrate limitation, low rumen pH, and specific bioactive fatty acids. The biohydrogenation theory became one of the more widely accepted explanations for milk fat depression. Since the discovery of certain fatty acid isomers that induce milk fat depression more investigations have aimed at how different nutrients interact in the rumen and affect the production of these milk fat inhibiting isomers. Readily available carbohydrates such as sugar, starch, neutral detergent soluble fiber, and rumen availability of these carbohydrate fractions, all effect the microbial populations within the rumen that are involved in the process of biohydrogenation. This review summarizes the knowledge available on milk fat depression, microbial influences, how soluble carbohydrate fractions influence on biohydrogenation.
Keywords
Milk fat depression; Starch; Soluble fiber; Biohydrogenation; In vivo; In vitro
Introduction
Inclusion of lipids into lactating dairy cow diets is a challenge due to the demand to meet the needs of the animal as well as the complexity of the rumen. Milk fat depression (MFD) is a centuries-old metabolic syndrome characterized by sustained drops in milk fat percentage. Milk fat is highly sensitive to changes in diet, and has been extensively investigated in ruminants. In many species, the fatty acid composition of milk fat reflects the fatty acid profile of the diet [1]. However, ruminants are unique in that the lipids consumed, depending on physical and chemical properties, will be transformed by bacterial metabolism in the rumen. Diet can alter the bacterial populations and rumen fermentation, which can have major effects on the fat content and fatty acid composition of milk.
Dietary, metabolic, and microbial changes due to milk fat depression have been investigated thoroughly and great strides have been made in the last 20 years in determining risk factors for the onset of the disorder. Recent work by Rico et al. [2] characterized the time course of induction and recovery from MFD, allowing researchers to have a greater understanding of the time required for MFD to emerge as well as how long it will take for farmers to recover milk fat to normal levels. Elucidating the onset of MFD and dietary modifications that can be made to recover milk fat is paramount to better understand the multitude of factors that may contribute to the disorder. Microbial population changes during MFD have been well documented, with these alterations being highly sensitive to changes in the ruminal synchrony of nutrients. Some research has focused on the utilization of sugars, neutral detergent fiberrich byproducts, and processed grains to increase the productivity of lactating cows [3-5]. However, some of these investigations observed some interesting effects related to milk fat production. For example, Martel et al. [4] reported that biohydrogenation was enhanced and rumen pH was increased with the inclusion of molasses in the diets of lactating dairy cows fed high-energy diets. There is evidence that sugar may decrease proportions of polyunsaturated fatty acids (PUFA) and trans 18:1 fatty acids in milk fat and increase milk fat content through the modulation of ruminal pH and fatty acid biohydrogenation pathways [6-8]. Sources high in neutral detergent soluble fiber have also been utilized for their economic benefits as well as their positive impacts on rumen fermentation. With the demand for corn driving the use of byproducts, research on feedstuffs such as soy hulls, citrus pulp, and beet pulp has increased. Voelker et al. [9] replaced a portion of the corn with beet pulp and observed a linear increase in milk fat. The soluble fiber in these byproducts are a source of rapidly fermentable energy that has been reported to yield more acetate and increase proliferation of cellulolytic microbiota, which may favor increased biohydrogenation and reduction of trans intermediate production in MFD scenarios [10]. Replacing a portion of the starch with sugars or beet pulp may improve production, but the degradability of the starch fraction is an important factor to consider as well. The structural organization of corn is likely the cause of decreased digestibility of whole shelled corn, and attempts have been made in order to increase starch availability, such as steam flaking. Ground high moisture corn and steam flaked corn have been reported to increase milk yield and protein percentage [11]. However, there has been some evidence that increased starch degradability yields greater proportions of trans intermediates [12,13]. Therefore, it is of interest to investigate ruminal and whole-animal responses to changes in dietary sugar, starch degradability, and neutral detergent soluble fiber with respect to preventing or ameliorating milk fat depression.
Fat in Ruminant Diets
Fat is commonly added to rations as a means to increase the energy density of the diet, improve palatability and reduce feed dust, and more recently, to alter the fatty acid (FA) profile of the meat or milk end product [14]. However, it is widely accepted that all fats are not created equal with regards to ruminants. Grass and corn silages contain 1-3% total FA, and grains and byproducts vary in their FA content depending on processing [15]. The amount of FA within plant species is highly dependent upon maturity at harvest, season, environment, and a variety of other factors. Silages and preserved forages make up a significant part of a dairy cow ration and are commonly added along with commercial fat supplements.
Triglycerides, galactolipids, and phospholipids are the three predominant glycerol-based lipids in animal feed ingredients [16]. Galactolipids make up a major portion of the glycolipids within forages and consist of a glycerol backbone with a bound galactose molecule and two FA [17]. Triglycerides are the major storage lipid in oilseeds, and therefore are greater in concentration in concentrate feedstuffs. Addition of fat to rations is common practice in order to increase dietary energy density without the risk of feeding excessive amounts of fermentable carbohydrates [18]. However, when added to a ration at a high inclusion rate, supplemental fat can have negative effects on dry matter intake, milk yield, and milk components [19].
The ideal fat is one that has no effect on rumen fermentation, has high intestinal digestibility, is easy to handle and mix into the total mixed ration (TMR), and increases milk and milk component yields. This is an inherent problem with unsaturated fatty acids, as they are highly modified by microbiota once they enter the rumen, so what goes in the system might not be the same post-rumen. Attempts to protect unsaturated fatty acids from microbial action have been developed by forming calcium salts, which minimize ruminal transformation of unsaturated fatty acids and risk of a decrease in fiber digestibility [16]. The discovery of these calcium salts could be considered somewhat of an accident. In an experiment conducted in the late 1970s by Dr. Don Palmquist the animals experienced a bout of milk fever that required the supplementation of calcium to alleviate the problem [20]. After analyzing the data it appeared that adding calcium to the diets improved the utilization of fat as well as the entire ration, thus beginning the investigation into developing calcium salts of fatty acids. These calcium salts are considered “bypass” or “rumen inert” fats and are formed by a reaction of divalent cations of Ca with long chain fatty acids (LCFA) to form insoluble fatty acid complexes [21]. Many of the commercially available Ca-salt FA supplements are derived from palm FA distillate and contain both saturated and unsaturated FA [22]. Some other ways of protecting fatty acids include encapsulation and hydrogenation. Natural encapsulation of fatty acids is also a form of protection in certain feedstuffs such as whole oilseeds, as the fatty acids are protected by the tough seed coat. However, disruption of this seed coat by mastication or disruption in the feed mixer can lead to the fatty acids being exposed and increases the risk of microbial transformation.
Mohamed et al. [23] reported that milk fat percentage was reduced when soybean or cottonseed oil were included in the diet, whereas whole cottonseed or whole soybeans did not reduce milk fat. This suggests that feeding whole oilseeds are less likely to lead to the onset of diet-induced MFD as compared to feeding free FA as oils. Other forms of encapsulation have been reported in the literature and include physical and chemical modifications. For example, encapsulation by combining unsaturated FA with casein and rendering it inert by cross-linking with formaldehyde or encasing the unsaturated FA within a saturated FA shell [24].
Saturated fats are a popular choice among dairy producers and nutritionists due to their versatility in how they can be added to rations without risk of causing milk fat depression (MFD). Saturated fatty acids have been shown to positively affect milk yield without decreasing dry matter intake and also have earlier resumption of ovarian activity, resulting in decreased days open [25]. Two of the most common saturated fatty acids in many fat products are palmitic (C16:0) and stearic (C18:0) acid. Although these fatty acids differ only in two carbons, they have unique and specific roles in the dairy cow. Palmitic acid is found in greater quantities in adipose tissue, whereas stearic acid is the predominant fatty acid absorbed by the mammary gland in the lactating dairy cow [26]. Levels of C18:0 are greater at the intestinal level than what was originally fed due to complete biohydrogenation of unsaturated fatty acids within the feed. Feeding saturated or unsaturated FA can supply necessary energy to the animal, but even excess saturated FA has been reported to depress fiber digestibility [18,27].
Rico et al. [19] investigated the effects of feeding a high-C16:0 or Ca-FA supplement in lactating cows and found that the supplement high in C16:0 increased DMI, milk yield, milk fat, and energy corrected milk (ECM) in comparison to the Ca-FA supplement that was higher in unsaturated fatty acids. However, reports of increased DMI or milk yields with feeding palmitic or stearic acid have been variable in some investigations [28,29]. Dietary FA profiles have a direct influence on milk fat concentration and yield. For example, abomasal infusion of short and medium chain saturated fatty acids increased the milk fat concentration and yield [30]. Strategic feeding of saturated FA for increased milk yields and energy density of the diet have also been under scrutiny for the impacts on milk products and consumer acceptability. A review by Livingstone et al. [31] suggests that consumption of dairy products where saturated FA were replaced with PUFA or MUFA improved plasma lipid markers of cardiovascular disease risk in humans.
Polyunsaturated FA, particularly of the n-3 family is of interest in human nutrition lately, due to their potential health benefits. However, the incorporation of PUFA or MUFA into milk fat is confounded by the complexity of nutrient interactions in the rumen as well as desaturation that occurs within the mammary gland. For example most of the cis-9, trans-11 conjugated linoleic acid (CLA) found in milk is primarily derived from the desaturation of trans-11 18:1 in the mammary gland itself [32]. The synthesis of milk fat and the origin of milk fatty acids will be discussed further in the next section.
Origin of Milk Fat
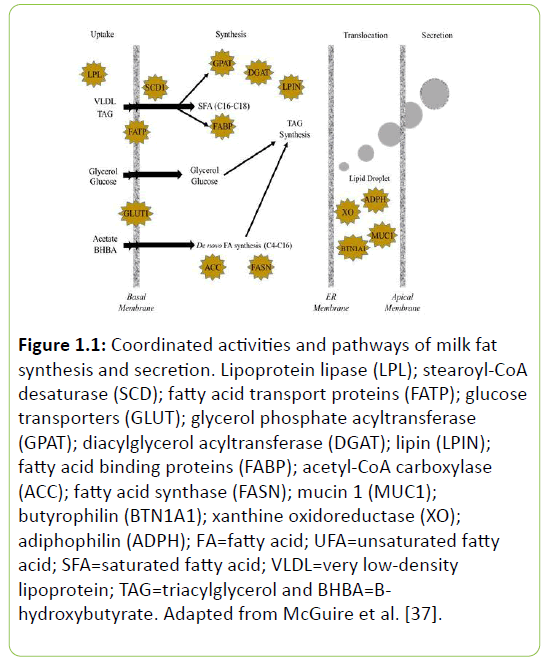
In order to understand dysfunction of mammary lipid synthesis, it is important to first understand the composition of milk and physiology of the mammary gland. It has been reported that milk contains over 400 fatty acids, of which 98% of the lipid is in triacylglycerol (TAG) form with three fatty acids esterified onto a glycerol-3-phosphate backbone [33]. Fatty acids within bovine milk fat are derived from two sources: they are synthesized de novo by the mammary gland, or are incorporated into the milk fat globule from plasma lipids originating from the feed or mobilization of body fat. De novo and preformed fatty acids differ significantly, with the de novo fatty acids ranging 4:0 to 14:0 in chain length, and preformed consisting of FA of greater than 16 carbons (Figure 1.1) [34]. Fatty acids of 16 carbons originate from both sources, as demonstrated by Palmquist et al. [35], who fed or intravenously infused 1-14C palmitic acid into lactating cows. De novo fatty acid synthesis has been reported to contribute approximately 45% of the total fatty acids in milk [36].
Figure 1.1: Coordinated activities and pathways of milk fat synthesis and secretion. Lipoprotein lipase (LPL); stearoyl-CoA desaturase (SCD); fatty acid transport proteins (FATP); glucose transporters (GLUT); glycerol phosphate acyltransferase (GPAT); diacylglycerol acyltransferase (DGAT); lipin (LPIN); fatty acid binding proteins (FABP); acetyl-CoA carboxylase (ACC); fatty acid synthase (FASN); mucin 1 (MUC1); butyrophilin (BTN1A1); xanthine oxidoreductase (XO); adiphophilin (ADPH); FA=fatty acid; UFA=unsaturated fatty acid; SFA=saturated fatty acid; VLDL=very low-density lipoprotein; TAG=triacylglycerol and BHBA=Bhydroxybutyrate. Adapted from McGuire et al. [37].
De novo fatty acid synthesis in the mammary gland utilizes mainly acetate and some β-hydroxybutyrate derived from rumen microbial fermentation (Figure 1.1). Once in the mammary gland, acetate is activated to acetyl-CoA by acyl-CoA synthetase short-chain family (ACSS), then carboxylated to malonyl-CoA, which is catalyzed by acetyl-CoA carboxylase (ACC) [38]. Malonyl-CoA then undergoes a chain elongation process by addition of two CH2 groups at a time catalyzed by the enzyme complex, fatty acid synthase (FAS) [39,40]. These fatty acids are straight-chain and possess an even number of carbons. However, propionate, valerate or isobutyrate can be utilized for fatty acid synthesis and result in the formation of branched-chain or oddnumbered carbon fatty acids [41]. The process of de novo synthesis requires reducing equivalents of nicotinamide adenine dinucleotide phosphate (NADPH), which are generated from the pentose phosphate cycle and the oxidation of isocitrate by isocitrate dehydrogenase [38].
The TAG-rich chylomicrons and VLDL of plasma are the primary source of preformed fatty acids taken up by the mammary gland, a process which is mediated by lipoprotein lipase [40]. Nonesterified fatty acids (NEFA) bound to albumin that is derived from the GI tract or mobilizations of body fat stores have also been reported to be a source of preformed FA [42]. The contribution of FA in milk from mobilized fat stores accounts for less than 10% of the FA in milk, but during periods where cows have a negative energy balance the contribution increases [34]. Fatty acids can also be desaturated within the mammary gland by stearoyl-CoA desaturase (SCD), which plays an important role in the modulation of unsaturation of membranes and TAG composition [38]. Palmitoleoyl-CoA, oleyl- CoA, cis-9, trans-11 and trans-7, cis-9 CLA isomers have been reported to be derived in part from SCD activity [32,42-45].
The TAG in milk is synthesized within the endoplasmic reticulum of the mammary epithelial cells by a variety of enzyme complexes. Fatty acyl-CoAs from de novo synthesis as well as preformed FA are esterified to a glycerol-3-phosphate backbone, which is derived from glycolysis or, less frequently, by phosphorylation of free glycerol by glycerol kinase [46]. Fatty acids are not distributed randomly on the sn-1, sn-2, and sn-3 positions on the glycerol backbone, which allows for FA to illicit functional or nutritional activities. Approximately half of the FA esterified at sn-1 and sn-2 are medium and long-chain saturated FA, and 44% of the FA at the sn-3 position is short-chain FA or oleic acid (27%). Fatty acids are esterified at the sn-1 position first by glycerol-3-phosphate acyl transferase (GPAT), which serves as the first committed step in TAG biosynthesis. Lysophosphatidic acid acyltransferase (LPAAT) catalyzes the second step with a greater preference for saturated FA (preference in order of C16>C14>C12>C10>C8) at the sn-2 position. Diacylglycerol acyltransferase (DGAT) esterifies both short and long chain fatty acids at the sn-3 position, and seems to be an enzyme complex unique to TAG synthesis [47]. The composition of milk fat can be modified by a variety of means including dietary changes, season, and physiological state. Milk fat from cows with milk fat depression has an altered FA composition, which will be discussed further in a later section.
Microbial Metabolism of Fats
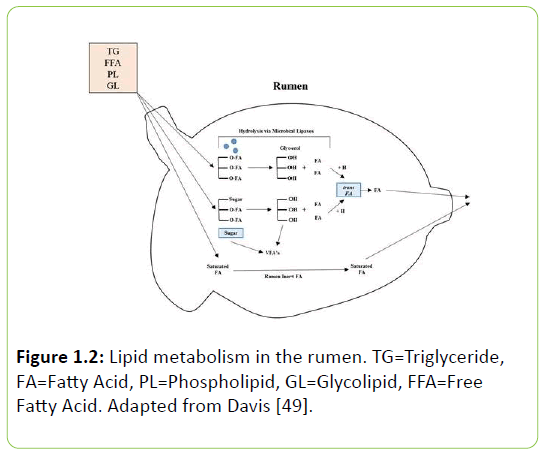
The microorganisms within the rumen are responsible for a multitude of symbiotic functions to the animal. Unsaturated fatty acids are considered antimicrobial agents in that they incorporate into the bacterial cell and can disrupt its activity [48]. Many of the feeds that a cow consumes consist of naturally occurring unsaturated plant lipids that the rumen microbiome must saturate. Rumen bacteria utilize a defense mechanism that allows them to convert PUFA to saturated fatty acids. Unsaturated fatty acids enter the rumen and undergo two important transformations elicited by ruminal microorganisms: hydrolysis and biohydrogenation. Hydrolysis is the separation of FFA from ester linkages by microbial lipases (Figure 1.2), which are extracellular enzymes packaged in membranous particles [41]. The released fatty acids then undergo biohydrogenation, a process which requires a free carboxyl group for its initial step [15].
Figure 1.2: Lipid metabolism in the rumen. TG=Triglyceride, FA=Fatty Acid, PL=Phospholipid, GL=Glycolipid, FFA=Free Fatty Acid. Adapted from Davis [49].
Biohydrogenation is a process bacteria use to add hydrogen’s to unsaturated fatty acids in order to saturate them. This protective mechanism is one of the primary reasons why the amounts of unsaturated fatty acids that enter the rumen do not equal the same quantity post-ruminally. There is a small amount of saturated FA that is incorporated into microbial membranes, but no fermentation of FA occurs.
The rate and extent of biohydrogenation is dependent upon rumen conditions such as pH and microbial populations. Instances such as low pH can result in incomplete biohydrogenation, leading to increased production of trans fatty acids [16]. Biohydrogenation of unsaturated fatty acids by ruminal microorganisms pose a specific problem for unsaturated fatty acid incorporation into tissues and milk. The mechanism of biohydrogenation could be considered a defense mechanism in that it enables unsaturated fatty acid-sensitive bacteria a way to tolerate the natural unsaturated fatty acids within plant tissues. Other factors such as total unsaturated fatty acid load within the rumen can influence the rate and extent of biohydrogenation and alter what fatty acid intermediates reach the small intestine. For example, fish oil supplementation alone or in combination with unsaturated plant oils have been shown to increase the amount of biohydrogenation intermediates escaping the rumen, particularly those with trans double bonds [50].
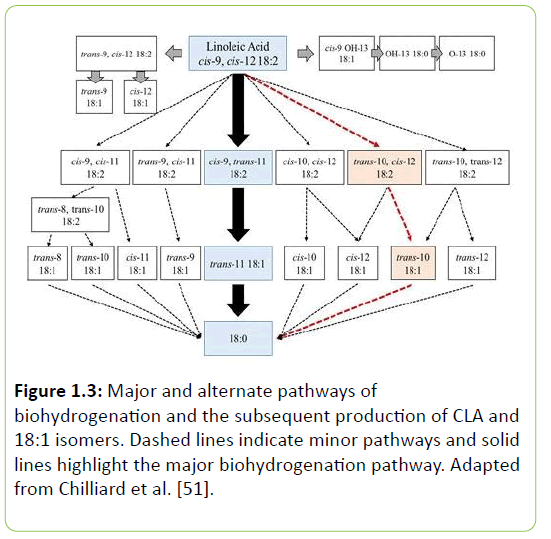
The biohydrogenation of linoleic and linolenic acid involves a major pathway, but there are also many intermediates that can be produced through alternate pathways. This is one of the reasons why there are many different isomers of conjugated linoleic acid that are derived from alternate pathways of biohydrogenation. These alternate pathways can also contribute to CLA isomers that have also been implicated in the reduction of milk fat synthesis. Figure 1.3 illustrates the alternate pathways that are involved in the biohydrogenation of linoleic acid.
Figure 1.3: Major and alternate pathways of biohydrogenation and the subsequent production of CLA and 18:1 isomers. Dashed lines indicate minor pathways and solid lines highlight the major biohydrogenation pathway. Adapted from Chilliard et al. [51].
Microbial species involved in fatty acid biohydrogenation are well documented. Most notably, Harfoot et al. [52] described two classes of bacteria that are involved in biohydrogenation; group A and group B. Group A bacteria can isomerize and hydrogenate 18 carbon fatty acids to monounsaturated fatty acids (MUFA), specifically octadecenoic fatty acids. Group B bacteria are then able to hydrogenate and saturate the cis double bond to stearic acid. Under normal circumstances, group A bacteria isomerize linoleic acid primarily to cis-9, trans-11 CLA then hydrogenate to cis-9, trans-11 CLA to trans-11 C18:1. Group B bacteria then hydrogenates trans-11 C18:1 to stearic acid, thus fully saturating the original PUFA if allowed to go to completion.
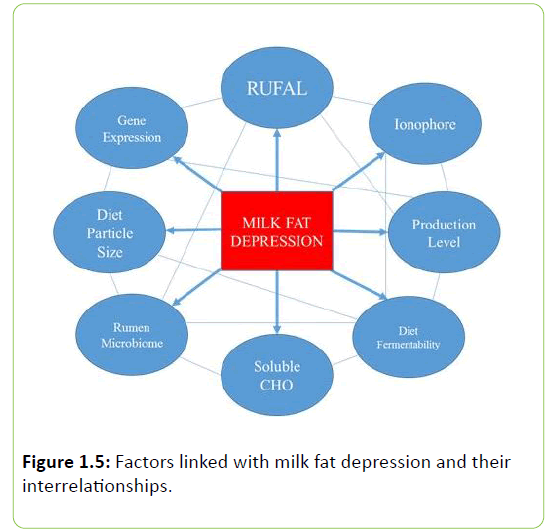
It has been observed that apart from diets high in PUFA, high concentrate rations, particle size, and rumen unsaturated fatty acid load (RUFAL) can all contribute to milk fat depression due to their interactions with these groups of bacteria. Rumen unsaturated fatty acid load is a term used to describe the total unsaturated fatty acid supply entering the reticulo-rumen each day from feed and is calculated as the sum of oleic, linoleic, and linolenic acids.
It has been shown in several investigations that milk fat depression is most commonly induced due to excess PUFA, thus leading to a high RUFAL. Microbial populations within the rumen are quite sensitive to unsaturated fatty acids, and attempt to “detoxify” them by the process of biohydrogenation. Several studies have identified specific bacterial populations that are prevalent during conditions of milk fat depression.
Lantham et al. [53] reported an increase in the relative abundance of Peptostreptococcus and a decrease in Butyvibrio spp. during low-roughage induced milk fat depression. Thus, providing an early example of microbial changes that occur during MFD. More recently, Megasphaera elsdenii, a lactate utilizing bacterium, was reported to account for up to 4% of the 16S rRNA gene copy number of the total microbial community in cows experiencing MFD [54]. However, Weimer et al. [55] failed to induce MFD by dosing M. elsdenii that was isolated from the rumen. This result was not surprising in that the establishment of exogenous bacterial strains in the rumen is challenging, even if the bacteria was isolated from the donor cow.
Maia et al. [48] observed that Butyrivibrio fibrisolvens produced primarily vaccenic acid in the presence of linoleic acid. The stearate producing bacterium Clostridium proteoclasticum did not grow in the presence of PUFA, indicating this population may be inhibited during MFD. Furthermore, Boeckaert et al. [56] noted that there was an increase in the trans C18:1 FA in the rumen that was related to changes populations of Butyrivibrio spp.
Kepler et al. [57] demonstrated early on that populations of Butyrivibrio fibrisolvens were able to hydrogenate trans-10, cis-12 CLA to trans 10 C18:1, and therefore may play a major role in MFD. Other reports have shown that Megasphaera elsdenii and Priopionibacterium produce trans-10, cis-12 CLA and could also play a role in the onset of MFD [58,59].
Protozoa have been implicated to have an impact on biohydrogenation in the rumen as well. However, this has been debated for quite some time. Keeney [60] reported that protozoa make up approximately 75% of the total microbial FA contribution, and that they are an important source of PUFA, CLA, and trans-11 C18:1 [4,50]. Early observations noted an almost complete absence of rumen protozoa during period of MFD, and the logical assumption was that protozoa contribute to biohydrogenation [61].
Chalupa et al. [62] showed that holotrich protozoa, even at double the concentration normally found within the rumen, were unable to hydrogenate linoleic-1-14C acid. Later works revisited the protozoal role in biohydrogenation and concluded that protozoa do not directly synthesize CLA or trans-11 C18:1, but contribute to the slow of FA biohydrogenation by maintaining a ruminal pool of FA within their membranes that is unavailable to biohydrogenating bacteria [4,63].
Anaerobic fungi make up a smaller part of the (estimated at 8%) total microbial mass in the rumen, but produce a wide variety of fibrolytic enzymes that are essential for forage fermentation [52,64]. Therefore, it is logical to assume that they may play a role in biohydrogenation as well. Nam et al. [65] tested this hypothesis and found that ruminal fungi do hydrogenate linoleic acid, but at a much slower rate. It was also observed that cis-9, trans-11 CLA was an intermediate and trans-11 C18:1 was the end product, with Orpinomyces comprising the most active role of the fungal isolates tested. Due to fungi representing only a small portion of the microbial biomass, it can be concluded that biohydrogenation by rumen fungi do not substantially contribute to the overall biohydrogenation capacity of the rumen. However, it is important to note that bacteria are not the only microbial domain in the rumen that can hydrogenate certain FA.
Conjugated Linoleic Acid
Conjugated linoleic acids are natural components derived from rumen transformation of linoleic acid or desaturation in the tissues, and represent a group of positional and geometric isomers of linoleic acid (Figure 1.2; cis-9, cis-12 octadecadienoic acid). The term “conjugated” refers to a fatty acid with two double bonds separated by one single bond. It has been reported that there are more than 20 different types of known CLA produced in the rumen by its microbial communities, but MFD has only been induced consistently by three [66].
These CLA milk fat inhibitors (CLAMFI) are absorbed and transported to the mammary gland, where essential enzymes for milk fat synthesis are inhibited, reducing the synthesis of milk fat. Milk contains moderate levels of CLA, and recent research has aimed to increase the CLA content in milk due to the anticarcinogenic properties of the cis-9, trans-11 CLA isomer. Of the 20 different isomers of CLA, cis-9, trans-11 (rumenic acid) is considered to be the most common and abundant form of CLA. Approximately 17 of these CLA isomers have been identified in cattle ruminal contents [67].
In a study by Lee et al. [67], a stable isotope of linoleic acid was incubated in rumen contents for 48 hours, resulting in the formation of several CLA isomers, including trans-10, cis-12 CLA. This study was aimed at identifying CLA isomers that are derived from the biohydrogenation of linoleic acid.
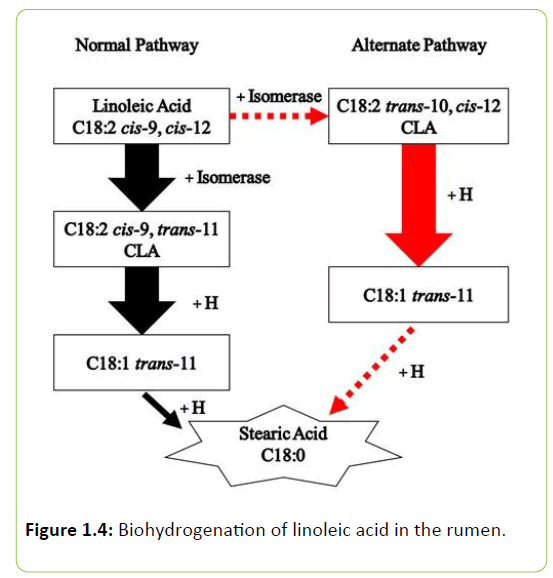
In the normal biohydrogenation pathway, the cis-9, cis-12 double bond complex of linoleic acid is initially isomerized at the cis-12 position to form cis-9, trans-11 CLA (Figure 1.4). The cis-9, trans-11 CLA is then converted to trans-11 C18:1 by the addition of a hydrogen atom. Conjugated linoleic acid in milk and meat of ruminants can be derived from two sources, either from ruminal biohydrogenation of linoleic acid or synthesis from trans-11 C18:1 by the animal’s tissues.
The trans-11 C18:1 CLA can then be converted into cis-9, trans-11 CLA in the tissues by Δ-9 desaturase. Delta-9 desaturase activity is greatest in the mammary gland of lactating cows, and cis-9, trans-11 CLA can be endogenously synthesized at this level. When animals were abomasally infused with sterculic acid, milk fat content was reduced in lactating cows, thus indicating a relationship between sterculic acid and inhibition of Δ9 desaturase [34].
In the rumen, CLA is an intermediate of the linoleic acid to stearic acid biohydrogenation. The previously outlined scenario is the normal pathway of biohydrogenation that these FA undergo. However, there is an alternate pathway that can produce trans-10 C18:1, and the potent milk fat inhibitor, trans-10, cis-12 CLA (Figure 1.4). The normal pathway can shift to the alternate biohydrogenation pathway due to a variety of factors including excess RUFAL, high grain diets, or poor effective fiber, for example [68]. The trans-10, cis-12 isomer has been denoted as the most potent inhibitor of synthesis of milk fat [69]. In a study conducted by Baumgard et al. [69], abomasally infused trans-10, cis-12 CLA resulted in a curvilinear reduction in milk fat yield occurred with increasing amounts of the isomer. Other isomers have also been reported to depress milk fat yield. This is the case for cis-10, trans-12 CLA which was reported by Saebo et al. [70] to induce MFD. Trans-9, cis-11 CLA was also shown to increase when milk fat was reduced through chromatographic analysis of milk samples from milk fat depressed cows [71]. Perfield et al. [72] later confirmed this finding by using a CLA enrichment that supplied 5 g/d of trans-9, cis-11 CLA via abomasal infusion reduced milk fat yield by 15%.
Adding plant-derived oils to an animal’s diet increases the milk fat concentration of CLA, but also may inhibit rumen microbial growth [34]. Conditions such as low forage, low fiber diets and/or increased intake of plant oils have decreased milk fat secretion in dairy cows. Streptococcus and lactobacillus bacteria have been shown to produce trans-10, cis-12 CLA [48]. Changes in milk fat composition were usually paired with a reduction in fatty acids derived from Δ9 desaturase activity. Moreover, restriction of feed intake has been reported to increase milk fat CLA from mobilized body fat stores [73].
Milk Fat Depression
Protein and fat are two of the most valuable components in milk. The concentrations of these major components can be widely altered by a variety of factors including genetics, health status, and nutrition. Nutritional changes can affect the milk fat content positively or negatively, with high concentrate diets sometimes exerting negative effects. Milk fat depression was first reported by French chemist Jean Baptiste Boussingault in 1845 who observed that when beets were fed to dairy cows butterfat was reduced [34]. Boussingault attributed this phenomenon to the inherently low fat content of the diet. This idea was not unrealistic in theory, since under MFD conditions the mammary gland may not have sufficient FA supply for lipogenesis. However, ruminants cannot directly convert glucose to FA, and acetate and butyrate are utilized by the mammary gland for de novo synthesis and long-chain FA derived from the diet or adipose tissue mobilization, are secreted in the milk [17]. The specific cause of low milk fat syndrome continued to elude producers for many years and prompted investigations into strategic feeding practices in the last century to determine the cause of MFD [74-76]. In the early part of the 21st century, MFD was observed with a variety of diets including cod liver oil [77], plant oils [78], and diets with high concentrates [79]. Davis et al. [80] divided diets that cause MFD into two groups, the first comprising diets with large amounts of readily digestible carbohydrates and reduced fiber, whereas the second group includes diets with high amounts of PUFA. There are many diets that may fall into either category, but a common denominator in the onset of MFD revolves around management practices, other dietary components, or the physiological state of the animal. Therefore, the separation of diets that cause MFD into two groups does not adequately assess the risk for MFD.
Acetate and Butyrate Theory
Many theories have been postulated to explain the MFD phenomenon. One theory suggests that inadequate fermentation in the rumen results in a reduced amount of lipogenic precursors, such as acetate and butyrate, causing a subsequent reduction in milk fat synthesis [34]. It is logical to infer that changes in rumen VFA production may be responsible for a reduction in milk fat due to the role of acetate and butyrate in milk fat synthesis. This precursor-product-ratio theory was first introduced by Tyznik et al. [81] and is based on the premise that reduction of ruminal acetate and butyrate production can in turn inhibit synthesis of milk fat due to a substrate shortage for de novo FA synthesis. Changes in VFA patterns have been reported with MFD, but the changes are most closely associated with the forage to concentrate ratio (F:C) or with low amounts of effective fiber. Acetate has been shown to be markedly reduced in these scenarios, but this is mainly due to the increase in relative ruminal propionate production. Milk fat depression has been reported to occur in diets supplemented with oils without the presence of any changes in ruminal VFA concentrations [81]. This theory was disproved in a number of studies, where acetate was infused yet milk fat concentration remained unchanged. Van Soest [17] noted that historically the acetate deficiency theory was undoubtedly disproved by McClymont [82], who found that glucose or propionate infusions produced low milk fat. However, acetate appears to alter milk fat in normal conditions when MFD is not present. In addition, Van Soest [17] expressed concern over the expression of VFAs as molar proportions without the total acid concentrations as well. Van Soest states that VFA proportions are of biological significance, but the value of the measurement is inherently skewed by the fact that changes in VFA concentration of one acid require a statistical change of the opposite sign in the other acids. In addition, it was due to this discrepancy that the “erroneous theory” that an acetate deficiency causes MFD. Further investigations into the exact cause of the onset of MFD continued after the abandonment of this theory.
Glucogenic-Insulin Theory
Mcclymont et al. [83] proposed a different theory, suggesting that high ruminal propionate concentrations and rates of hepatic gluconeogenesis can result in an increased circulating insulin spike, and thus, precursors available for milk fat synthesis might be limited due to an insulin-induced response. This theory gained traction due to the logical assumption that milk fat depression from high-concentrate feeding was due to increased ruminal propionate flux and subsequent stimulation of hepatic glucose synthesis. This would in turn increase the release of insulin. However, in the mammary gland of the ruminant, insulin is responsible for normal mammary cell function maintenance and is not affected by fluctuations in circulating meal-induced insulin spikes since it only requires relatively small quantities of insulin. The rate of lipogenesis and lipolysis in other ruminant tissues are affected by insulin, and may somewhat affect the nutrient availability to the mammary gland [34]. Furthermore, this glucogenic-insulin effect is thought to divert nutrients from the mammary gland due to the increase in circulating insulin levels, which result in the increase utilization of acetate, β- hydroxybutyrate, and dietary LCFA at the adipose tissue level. Therefore, there is a shortage of lipogenic precursors such as acetate and β-hydroxybutyrate for milk fat synthesis [34]. This theory has received considerable attention which prompted many investigations into its validity.
Davis et al. [80] summarized a number of trials that utilized infusions of propionate to test this theory, but reductions in milk fat yield were variable and the involvement of insulin in MFD remained unclear. Frobish et al. [84] infused glucose and propionate into the abomasum and no MFD was observed. The authors argued that the observations by McClymont et al. [83] were under physiological and that other feeding studies with propionate had shown MFD. Use of a hyperinsulinemiceuglycemic clamp has been tested in order to minimize disruptions in glucose homeostasis and hypoglycemia, but the effects on the synthesis of milk fat were minimal. The hyperinsulinemic-euglycemic clamp method works by raising and maintaining plasma insulin levels through a continuous infusion of insulin. When insulin levels hold steady, the glucose infusion rate equals glucose uptake by all the peripheral tissues. McGuire et al. [85] found that milk yield and milk fat yield remained unchanged during the insulin clamp study, yielding little support for the glucogenic-insulin theory.
Bauman et al. [86] reported that the changes in some of the insulin clamp studies were marginal, reporting milk fat yield reductions of less than 5% during some of these investigations. Corl et al. [87] reported a decrease in milk fat percentage and yield, but determined that this was a result of the ability of insulin to inhibit lipolysis and limit preformed fatty acids available to the mammary gland. Furthermore, insulin plays a major role in the regulation of lipolysis through metabolic triggers that mobilize fat stores or signal adipose to store FA and start lipogenesis based on insulin levels. However, in regards to the glucogenic-insulin theory it is believed that the differences in insulin clamp studies with respect to MFD were primarily due to energy balance [85,88,89]. This is further supported by Corl et al. [87], who determined that the observed reduction in milk fat could be attributed to the mobilization of body fat reserves due to the experimental cows being in very early lactation. Bauman et al. [66] reported that during a time of negative energy balance, MFD repartitions the energy available toward protein and milk synthesis. This critical period result in greater mobilization of FA to meet energy demands of the mammary gland as well as replenish body reserves, thus, the reduction in milk fat reported in these studies cannot be attributed to the glucogenic-insulin theory but rather energetic balance.
Vitamin B12/Methylmalonate Theory
Similar to the previously discussed theory, Frobish et al. [84] presented the vitamin B12/methylmalonate theory of MFD. Vitamin B12 is an essential component of the enzyme methylmalonyl CoA mutase, which plays a major role in propionate metabolism [86]. The concept is centered on reduced production of vitamin B12, coupled with increased propionate, would cause an accumulation of methylmalonate within the liver that would then travel to the mammary gland and inhibit de novo FA synthesis through the down regulation of ACC and FAS. Often in cases where high concentrate diets are fed there is an observed increase in propionate and reduction in vitamin B12. Walker et al. [90] reported that high concentrate diets could potentially limit the amount of vitamin B12 produced in the rumen, and later studies suggested that it could also be the cause of MFD in dairy cows fed low forage diets. However, Elliot et al. [91] could not confirm these findings and observed that vitamin B12 injections did not alter milk fat. This was later confirmed by Croom et al. [92], where vitamin B12 failed to correct the decrease in milk fat production associated with feeding low forage diets.
Trans Fatty Acid Theory
The trans fatty acid theory involved the idea that milk fat synthesis is depressed by trans octadecenoic acid that is produced through incomplete and modified rumen biohydrogenation. This theory forms the foundation of the most current and widely accepted biohydrogenation theory and was first suggested by Davis et al. [80]. Davis et al. [80] observed an increase in C18:1 and postulated that this response was mostly due to the accumulation of trans C18:1 during MFD. This theory was expanded upon by multiple studies that showed that trans C18:1 increased in the milk fat of a variety of diets related to MFD [93-95]. Trans-11 C18:1 is an intermediate in the biohydrogenation pathway of linoleic acid (Figure 1.3) and linolenic acid. This FA is the most predominant trans octadecenoic acid isomer present in milk fat [33], so it was logical that an increase in this isomer during MFD would be the cause. However, investigations by Kalscheur et al. [96], and Selner et al. [97] demonstrated that there can be increased trans C18:1 due to dietary modification, and milk fat remains unchanged. Work by Griinari et al. [98] expanded upon this finding and reported that trans-10 C18:1 was to blame rather than all trans C18:1 isomers. Trans-10 C18:1 is also produced from the ruminal biohydrogenation of linoleic acid (Figure 1.4), and is a major part of a minor pathway later proposed by Griinari et al. [99] that helped to formulate the most current and widely accepted theory. The trans fatty acid theory was centered around the concept of trans C18:1 isomers as the cause of MFD, but there were too many inconsistencies and the identification of specific isomers in later work confirmed that the theory had conceptual flaws. Further investigations into the trans FA theory indicate that trans-10 C18:1 does not directly control milk fat production, but may respond to dietary factors [100]. However, the concentration of trans-10 C18:1 has been consistently reported to negatively affect milk fat yield and acts more as a proxy for the identification of milk fat depression [47].
Biohydrogenation Theory
The discovery of an anti-mutagenic compound in fried ground beef in the 70s by Mike Pariza [101-103], which was later identified as conjugated linoleic acid, shifted attention to increasing the content of CLA in dairy products due to the potential health benefits. In an attempt to increase milk fat CLA, it was observed that infusion of a CLA supplement that included a mixture of CLA isomers (predominately cis-9, trans-11 and trans-10, cis-12 CLA) severely reduced milk fat [104]. These investigations into enhancing milk CLA content gave a great deal of insight related to milk fat depression. After the discovery that CLA isomers inhibit milk fat production, investigations centered on determining which CLA isomer is responsible and identifying the proposed biohydrogenation pathway. Baumgard et al. [69] identified the potential culprit of MFD as the trans-10, cis-12 CLA isomer. Further work by Baumgard et al. [105] and Peterson et al. [106] showed that milk fat yield can be decreased by 40-50% with as little as 10 g/d of trans-10, cis-12 CLA. In addition, Baumgard et al. [105] characterized changes in milk fatty acid composition and reported a marked decrease in de novo fatty acids, a common observation seen with MFD. Griinari et al. [99] postulated the biohydrogenation theory in order to build upon the limitations of the trans FA theory and stated that under certain dietary conditions the pathways of rumen biohydrogenation change and produce fatty acid intermediates that can reduce milk fat synthesis. Work by Griinari et al. [98] confirmed that a dietary supply of PUFA and a change in microbial processes in the rumen were essential for diet-induced MFD. In addition, early work by Loor et al. [107] demonstrated that exogenous supply of trans-10, cis-12 CLA decreased milk fat yield and composition, and that trans-10, cis-12 CLA reduces de novo FA synthesis and desaturation in the mammary gland.
Practical Implications of MFD
The majority of dairy herds have been able to reduce the incidence of MFD because of sound nutritional management in their feeding program. However, even the best herd with the best nutrition program can be susceptible to MFD. Dietary ingredients available to dairy farmers are constantly changing depending upon price, availability, unexpected changes in nutrient composition, energy levels, and requirements to increase milk yield and components. Therefore, nutritional program manipulations can result in MFD within a short period of time. Dairy cows not exhibiting MFD syndrome also express CLAMFI, but at concentrations too low to cause MFD. Once MFD has been established, the identification of cause and respective amelioration can take several months. Under current milk pricing system, MFD represents a major concern for dairy producers. Based on the February 2018 milk fat price of $5.20/kg (USDA-FMMO), each 0.1% units decrease in milk fat results in a loss of $0.19/cow/d (based on 36 kg milk/d; average US milk production/d). A 200 cow herd that decreased from 3.6% to 3.3% milk fat would lose $114/d or $1140/d in a 2000 cow herd farm. Therefore, mitigating milk fat depression is very important to increase dairy farm profitability.
Milk fat depression is a multifactorial metabolic disorder, with onset being attributed to a number of conditions including, particle size, RUFAL, and rumen pH. It has been suggested that if pH is increased, CLAMFI will not be produced in such quantity as with low pH conditions. Jenkins et al. [108] observed an increase in pH coupled with a decrease in trans-10 18:1 and trans-10, cis-12 18:2 in continuous cultures supplemented with potassium carbonate. Although other factors may explain these effects, it is suggested that pH can improve rumen conditions and prevent the shift in CLAMFI production. When ruminal pH is decreased, bacterial species whose activity is primarily in the second reductase step in the biohydrogenation pathway are reduced, resulting in an accumulation of these intermediates [108]. Fuentes et al. [100] demonstrated that when culture pH was lowered from 6.5 to 5.5 there was an observed shift in CLA production that resulted in an increase in CLAMFI. However, Harvatine et al. [109] reported that MFD can appear even when pH is not reduced. This observation involves other contributing factors that can lead to the onset of MFD. Alterations of rumen microbial populations through dietary interventions to favor less accumulation of CLAMFI have been investigated thoroughly. Replacing starch with sugar sources has been shown to reduce the risk for MFD without disrupting performance [8]. Rico et al. [110] observed that altering the fermentability of the diet through modifying dietary NDF resulted in recovery from milk fat depression. However, it remains unclear whether or not these conditions will continue to be favorable when dietary changes in starch degradability or sugar inclusion, for example, are modified with a high PUFA diet.
Cellular Signaling of Fatty Acids
The theory that CLA isomers were responsible for reductions in milk fat was first introduced by Bauman [66]. Conjugated linoleic acid was first recognized for its anti-carcinogenic properties by Pariza in the late 70s and early 80s in fried hamburger [101-103]. However, the isomer associated with these effects, cis-9, trans-11 CLA, is one of many of bioactive isomers. Bioactivity refers to effects on downstream target genes in the mammary gland, and alterations in the microbial communities within the rumen. The isomer trans-10, cis-12, has been associated with milk fat depression, and it’s believed that it suppresses genes responsible for milk fat production in the mammary gland, such as stearoyl-CoA desaturase 1 (SCD1), fatty acid synthase (FASN), acetyl-CoA carboxylase (ACACA), and glycerol-3-phosphate acyltransferase 6 (AGPT6) [111,112]. This trans-10, cis-12 CLA isomer was first recognized as a potent inhibitor of milk fat production [69]. It has been reported that this isomer decreases body fat in chickens and rabbits, but has not altered body fat stores in ruminants, suggesting that it has a greater capacity for targeted cellular control than previously thought [113-115]. Although the mechanism by which CLA affects body fat is unknown, it is suggested that it causes a negative energy balance by reducing energy intake, and increasing energy excretion through heat loss [115]. This may be similar to what happens at the mammary level. Studies in mice, pigs, and sheep where trans-10, cis-12 CLA was supplemented showed a coordinated decrease in milk fat content [116-118].
Nutrigenomics applies high-throughput genomic methods in nutrition research, and has become an integral part of understanding the interactions between nutrients and tissue metabolism. Small changes in gene expression are able to be measured by using techniques such as real-time PCR and microarray, with the latter being of great importance in studying the transcriptome [119]. Recent advances in nutrigenomics have allowed researchers to characterize nutrient-tissue interactions, and quite possibly begin to explain the underlying mechanism of MFD [68,120]. The trans-10, cis-12 CLA isomer has been reported as the most potent inhibitor of milk fat synthesis, but trans-9, cis-11 CLA and cis-10, trans-12 CLA isomers have also exhibited inhibitory effects on milk fat synthesis [70,72]. Since fat is the only milk component affected by trans-10, cis-12 CLA, it appears that effects on the biochemical pathways involved in lipid synthesis in the mammary gland are highly specific [66].
Transcription factors play an intricate role in whether a particular gene is active, and interest in determining the “master regulators” involved in MFD have been investigated recently. Sterol regulatory element binding protein-1 (SREBP1; Figure 1.4) is highly active in mammary tissue. Although changes in gene networks associated with milk fat depression are not thoroughly understood, the activation of SREBP1 has been well described. In short, SREBP is associated with a chaperone protein known as SCAP, and forms a SREBP/SCAP complex within the ER, when inactive. Activation is accomplished through the dissociation of insulin-induced gene 1 or 2 (INSIG), which allows translocation to the Golgi. Once in the Golgi apparatus SREBP1 is proteolytically cleaved to form the active transcription fragment, nuclear SREBP1 (nSREBP1). This nSREBP1 transcription fragment is translocated to the nucleus where it can bind to sterolregulatory elements and eventually stimulate transcription of genes that are involved in the regulation of lipogenesis [121]. Despite SCAP having integral parts in lipid regulation, some investigations have reported only small differences in SCAP expression or abundance with CLA induced MFD [122]. However, INSIG1 was reported to be reduced with trans-10, cis-12 CLA treatment by Vyas et al. [123] and Harvatine et al. [122]. Furthermore, INSIG1 has been implicated in the amount of de novo and preformed FA in the mammary gland [41]. The apparent sensitivity of INSIG1 and lack of an effect on SCAP indicate that the alterations in lipogenesis may be regulated by downstream gene networks associated with SREBP1. Work by Peterson et al. [124] reported a decreased abundance of SREBP1 during trans-10, cis-12 CLA inhibition of FA synthesis in MAC-T mammary epithelial cell cultures. Further, Harvatine et al. [122] observed a decrease in expression of SREBP1 in mammary tissue from cows experiencing MFD. Down regulation of SREBP1, SREBP1 activation proteins and SREBP1-regulated genes expression for key enzymes involved in fat synthesis in bovine mammary epithelial cells suggests a high influence of SREBP1 as a central signaling pathway in the regulation of FA synthesis. Recent work has shown that the isomer trans-10, cis-12 CLA inhibits fatty acid synthesis through down regulation of genes involved in de novo fatty acid synthesis in goat mammary epithelial cells, and this process is likely correlated to the activation of AMP-activated protein kinase signaling pathways [121]. Furthermore, milk fat production is energetically expensive, so it is logical to infer that there may be an energy sparing effect during MFD. Harvatine et al. [125] delivered 7.5 g/d of trans-10, cis-12 CLA over the course of 4 d and observed a substantial decrease in milk fat yield and content, despite milk yield and other components being unaffected. One of the key findings in this study is that during MFD, adipose tissue expression of adipogenesis enzymes including lipoprotein lipase, FASN, SCD, and FABP4 were all increased. In addition, key regulators of lipid synthesis, SREBP1, thyroid hormone spot 14 (S14), and PPARγ were also increased in adipose tissue. These findings suggest that during milk fat depression there is an energy sparing effect from the reduction in milk fat synthesis and thus shuttled toward adipose tissue fat stores during MFD.
Soluble Carbohydrates and Starch Degradability
Carbohydrates represent 60-70% of the DM in dairy cow rations compared to other dietary nutrients [126]. Nonstructural (NSC) and structural carbohydrates are the two classifications of carbohydrates, with the nonstructural fraction consisting of sugars, starches, organic acids, fructans, and structural carbohydrates being the fibrous components. Nonstructural carbohydrates are highly fermentable, and oftentimes used in diets requiring high amounts of dietary energy, such as a lactating dairy cow ration. Nonfibrous carbohydrate (NFC) is a term that cannot be used in place of NSC, since pectin is included in NFC, but not in the NSC fraction. Organic acids make up a large difference in the NSC and NFC. Diet fermentability plays a major role in many of the nutrient interactions within the rumen. Highly fermentable diets have been implicated in the induction of dietary syndromes such as acidosis and milk fat depression. Work by Rico et al. [110] showed that milk fat synthesis increased significantly when diet fermentability and PUFA content was decreased. Other strategies have included the use of rumen modifiers to improve milk fat production. The methionine analog, 2-hydroxy-4-(methylthio)butanoate (HMTBa), has been shown to increase milk fat when feeding highly fermentable diets [127,128]. Baldin et al. [129] investigated the effect of diet fermentability and HMTBa supplementation and observed that cows with a high risk of biohydrogenation–induced MFD had greater milk fat with HMTBa. It was suggested that the HMTBa decreased the absorption of alternate biohydrogenation intermediates that are responsible for the onset of MFD [129]. As MFD is the result of the interaction of numerous factors, multiple dietary components have to be evaluated, as they have the potential to shift rumen microbial populations and alter the production of biohydrogenation intermediates.
Neutral Detergent Soluble Fiber
Sources high in starch, such as corn, or other grains, are common feedstuffs, and represent a large portion of energy that can be made available to the cow. However, starch can be quickly degraded and lead to acid accumulation and low rumen pH. Low rumen pH contributes to subacute ruminal acidosis (SARA), and can also result in shifts in the biohydrogenation pathway associated with milk fat depression. Therefore, replacing starch with other fermentable carbohydrate sources is of interest. The neutral detergent-soluble carbohydrate (NDSC) fraction is defined as a rapidly fermentable energy source for ruminal microbial growth and energy available for use by the cow. The NDSC fraction includes organic acids, monosaccharides, oligosaccharides, starch, fructans, pectin, β- glucans, and are not recovered in the NDF fraction [130]. Pectin and β-glucans are structural carbohydrates, but are soluble in neutral detergent solution, and are therefore classified as neutral detergent-soluble fiber (NDSF). Pectin is the predominant carbohydrate in NDSF, and possesses different fermentation and digestion than its NDSC fraction counterparts. Fermentation of pectin generates more acetate, less propionate, and lactic acid, with a greater rate of digestion when compared to NDF, but slower digestion than sugar or starch [131]. Sources high in pectin include legume forages, soybean hulls, beet pulp, and citrus pulp [132,133]. In some reports, replacing corn with non-forage fibrous byproducts increased total tract starch digestibility [134]. Even though NDSF is also fermented rapidly in the rumen, it is not fermented to lactate and will not continue fermenting when pH reaches a nadir [135]. Additionally, the use of sources rich in pectin have been shown to increase microbial protein synthesis, suggesting that NDSF can provide similar sources of energy compared with starch for supporting essential ruminal microbial growth [136]. Feedstuffs high in NDSF may help to improve animal production and the ruminal environment when challenged with a diet high in PUFA (Table 1). However, this has not been extensively investigated. In a recent investigation by Santos-Silva et al. [137] cereal grains were replaced with dried citrus pulp in high soybean oil diets fed to ewes it was observed that milk fat yield was unchanged, but milk yield was increased with citrus pulp. In a similar study conducted using lactating Holstein cows, Santos et al. [138] found that milk fat yield or composition showed no improvement with the addition of citrus pulp. However, both of the aforementioned studies aimed at investigating the effect on the milk or milk solids. Assis et al. [139] observed that a total replacement of corn with citrus pulp in lactating cows will not alter production, and Santos et al. [138] reported an increase in milk production where citrus pulp replaced corn up to 14% of the diet DM. In a review by Bradford et al. [140], it was noted that high-producing dairy cows may benefit from dietary inclusion of nonforage fiber sources. Butyrivibrio fibrisolvens has been reported to play a major role in the conversion of linoleic acid to CLA and then hydrogenation to trans-11 C18:1. This bacterium has starch and pectin degrading abilities, which may have major implications for diets high in NDSF and PUFA [141]. Moreover, Solomon et al. [141] observed that a high pectin diet with full fat extruded soybeans did not reduce milk fat. Thus, it is of interest to evaluate the effect of replacing a portion of starch with ingredients rich in NDSF.
| Soluble Carbohydrate | |||
|---|---|---|---|
| Item | Soluble Fiber | Sugars | Starch Kd |
| Milk Fat Yield | +bde, -f | +gk | No effectlmn |
| Milk Fat % | +bde, -f | +gk | No effectln, -ms |
| Milk Yield | No effectbc | No effectg, -k | +m |
| Biohydrogenation | +r | +jk | -1o |
| pH | +aq, -p | No effectg, +hk, -i | -1, +o |
| 1a=Strobel et al. [131]; b=Mansfield et al. [142]; c=Voelker et al. [9]; d=Van Knegsel et al. [143]; e=Shahmoradi et al. [144]; f=Boguhn et al. [145]; g=Broderick et al. [6]; h=Heldt et al. [146]; i=Kellog [147]; j=Ribiero et al. [148]; k=Martel et al. [4]; l=Harvatine et al. [149]; m=Theurer et al. [150]; n=Dann et al. [151]; o=Lascano et al. [13]; p=Naderi et al. [5]; q=Leiva et al. [132]; r=Santos-Silva et al. [137]; s=Bradford et al. [25]. | |||
Table 1: References that have investigated soluble carbohydrates and their effect on milk fat composition and yield, milk yield, rumen pH, biohydrogenation, CLA isomer production. Kd=starch degradability.1
Sugars
Sugars such as sucrose and molasses have been investigated in their influences on ruminal pH, VFA, biohydrogenation, and milk fat production (Table 1) [4,6,152]. Sugars can provide another source of energy the rations of lactating dairy animals that provide a rapidly degraded source of carbohydrates. There has been recent interest in using sugar to replace a portion of the starch due to the increasing demand and price of corn and starch sources [140]. Sugars can be beneficial in that they require little enzymatic activity to cleave the disaccharide and provide an energy source for the rumen microbes. It has been proposed that utilization of sugars in dairy rations could help prevent drops in pH and promote fiber digestibility [7,153,154]. This positive influence could minimize acidosis problems and limit the risk of milk fat depression. Martel et al. [4] reported that addition of 5% molasses in the diet can improve milk fat synthesis, increase ruminal pH, and increase ruminal biohydrogenation. The increase in ruminal pH is of importance due to the effects on milk fat inhibitors that are prevalent in periods of low ruminal pH. However, Oba [155] reported that a large majority of in vivo studies found no effect of sugar on pH, but that the few studies that do report increases in pH replaced a portion of the starch with sugar. The theory behind how addition of molasses can alter ruminal pH is suggested to be due to the production of butyrate. Molasses increases butyrate production, thus, stimulating blood flow and potentially increasing uptake rate of volatile fatty acids across the rumen epithelium. Guan et al. [156] observed that greater butyrate concentration in rumen fluid was associated with greater feed efficiency of beef cattle. Some in vitro studies have shown that sugar fermentation results in increased butyrate [157,148], but several in vivo studies were unable to replicate this finding [155]. Oba et al. [158] attributed this discrepancy due to the rapid fermentation of sugar immediately after consumption and because butyrate is absorbed faster than acetate or propionate and therefore changes may not be able to be detected. Furthermore, sucrose addition had greater molar proportions of butyrate and higher ruminal pH as compared to lactose [158]. The effects of feeding sugar and subsequent enhanced butyrate absorption on gut proliferation and overall nutrient metabolism in ruminants warrant further investigation. However, Oba et al. [158] also found an increase in the relative mRNA abundance of genes responsible for the facilitation of absorption and fermentation of ruminal VFA with dosing of sucrose or lactose. This observation sheds light on the increases in rumen pH due to enhanced ruminal epithelial permeability of VFA which has been observed despite the rapid fermentation rates of sugars. The responses seen with sucrose but not lactose are most likely due to the more rapid fermentation of sucrose. Sucrose is a disaccharide of glucose and fructose, while lactose is composed of glucose and galactose. The two disaccharides are similar in that each consists of one glucose monosaccharide, but they differ only by fructose and galactose. Weisbjerg et al. [159] reported that sucrose hydrolyzes at a much faster rate than lactose, and Sutton [160] noted that fructose and glucose ferment faster than galactose. Weisbjerg et al. [159] found that sucrose had a hydrolysis rate of 1200-1404%/h-1 and was not increased by addition to the basal diet, whereas lactose had a much lower rate of hydrolysis (248%/h-1) that was increased to 540%/h-1 when added to the basal diet. Some investigations have reported fructose increases the molar proportion of ruminal propionate, while lactose reduced propionate [161]. Propionate, a glucogenic precursor, can stimulate the release of insulin. There is some evidence that after sucrose administration propionate concentration is increased in the rumen and a concordant increase in plasma insulin when compared to starch or lactose dosing [162]. Furthermore, lactose has also been shown to increase rumen butyrate concentrations in some reports, with the increase potentially also causing some changes in ruminal epithelial tissue that results in greater VFA absorption [163]. This is logical in that rumen pH influences the rates of VFA absorption passive diffusion and protein mediated transport [17]. Chibisa et al. [163] found that lactose supplementation led to an increase in Cl- competitive absorption of acetate and propionate, which suggested that carrier-mediated transport of dissociated acetate and propionate was upregulated. The authors noted that this could have potentially increased HCO3- influx into the rumen and explains why they did not see a drastic drop in rumen pH with lactose administration.
Many investigations have aimed at replacing a portion of the starch with sugar and reported positive responses with pH, DMI, milk production, and milk fat yield [155,163]. Supplementing sugar sources high in sucrose, such as molasses, to lactating cows has been reported to result in increased DMI [6,7,152]. However, these reports in DMI response to sugar supplementation have been noted to vary due to the importance of nutrient synchronization with nitrogen, forage inclusion in the diet, and forage quality [152]. Sun et al. [164] found that replacing dietary starch with sucrose inhibited the trans-10 biohydrogenation pathway and may account for the positive effects seen in milk fat production in subsequent investigations. The effects of feeding high-sugar diets on milk fat production and energy metabolism have been consistently reported in the literature [155]. Several studies have reported that cows fed diets high in sugar increased [6,7] or tended to increase [165] milk fat yield. These responses may partly be attributed to reduced trans-fatty acid production in the rumen. Incomplete biohydrogenation of unsaturated fatty acids is a primary cause of milk fat depression [166], but Ribeiro et al. [148] demonstrated that biohydrogenation of unsaturated fatty acids decreased linearly with sucrose addition in continuous culture media. In agreement with their findings, Penner et al. [7] showed that feeding a high-sugar diet decreased C18:1-trans fatty acid concentration in milk fat, and tended to increase milk fat yield. There has been a recent interest in the addition of sugar to rations as a means to replace a proportion of the starch. This dietary modification can potentially help control rumen pH, particularly if part of the starch is replaced while maintaining constant NFC. In a recent study by Razzaghi et al. [167], sucrose was supplemented in combination with sunflower seed, and an increase in rumen pH was observed along with a tendency for a decrease in the concentration of total trans 18:1 FA. The interactions between fatty acids, starch, soluble fiber, and sugar need to be investigated in order to determine how they influence rumen fermentation, biohydrogenation, and animal production.
Amounts of NSC and NFC to be fed are not well outlined, with the concentrations depending on the interrelationships of starch on ruminal fiber digestion, site of starch digestion, dry mater intake (DMI), replacement of NDF with NSC or NFC, and the method of processing. The NRC [126] suggests the NSC concentration in a diet should not exceed 30-40 percent of ration (DM basis) and NFC 2 to 3% higher, with the latter difference being due to the inclusion of pectin within the NFC fraction. The amount of dietary NFC has an effect on fermentation parameters and ultimately milk fat percentage.
Starch Degradability
Starch accounts from 50 to 100 percent of the NSC in most feeds, and the rate of ruminal digestion is paramount in determining the amount of starch that can be added to a diet in safe quantities [126]. Processing plays an important role in starch degradability, with particle size reduction and heat processing being methods of altering the feedstuff and increasing or decreasing starch availability [126]. The interrelationships between nutrient fractions, such as starch, may play a pivotal role in the biohydrogenation of these unsaturated fatty acids and ultimately affect the quantity and type of fatty acids that escape to the duodenum. Ruminal microbes require a supply of fermentable carbohydrates for growth and protein synthesis. Starch provides a substrate for this microbial growth and greater ruminal degradability [168]. Increasing the degradability of starch in cereal grain sources has been investigated using various processing methods such as dry rolling and steam flaking [169]. Differing processing methods vary in degradability and fermentability. However, increased starch content and degradability may lead to low ruminal pH conditions, which affect biohydrogenation, animal performance, fiber digestibility, and microbial populations [170,171]. Hatew et al. [172] reported differing levels of starch degradability in situ can effect methane production. Increased starch degradability resulted in reduced enteric methane production, suggesting that fermentation of starch favors production of propionate, creating an alternative hydrogen sink for methanogens.
A study by Bradford et al. [25] characterized production responses to low forage diets that included dry ground corn or high moisture corn. The high moisture corn depressed milk fat content, but the authors recognized that cows producing over 45 kg/d of milk had a higher milk fat content with the high moisture corn diet than low producing cows. It was suggested that high producing cows have a greater capacity for biohydrogenation of FA and decreased delivery of milk fat inhibiting isomers to the mammary gland. This observation is interesting but it should be noted that the degree of processing and incorporation of the corn sources can vary, as well as production responses. There is extensive literature on processing methods for corn and small grains and the subsequent effects on milk production, animal performance, and digestibility of nutrients [150]. Ensiling of high moisture corn or steam treatment of dry corn, for example, breaks down the hydrophobic starch-protein matrix and increases starch digestibility [134]. Corn has one of the greatest starch densities of the cereal grains commonly fed to livestock, and as grain processing becomes more extensive the starch within is generally made more available for digestion [150]. Gelatinization is a process that is typically involved in the application of heat and moisture to the kernel in the amorphous region and later moving to the crystalline regions. Grinding corn when compared to dry rolling corn increases the mean particle size, thus, increasing starch digestibility and NEL by increasing the available surface area for bacteria to adhere to as well as opportunities for enzymatic degradation [11,173]. Steam flaking of corn or sorghum was reported to increase total milk yield and protein percentage, but decrease milk fat percentage [150]. Utilization of feedstuffs such as steam flaked or high moisture corn can help to improve flexibility and efficiency of dairy production, but should be carefully evaluated to ensure minimization of acidosis, depressed fiber digestibility, and potential reductions in milk fat production.
Ruminal Fatty Acid Metabolism using Continuous Culture Systems
Continuous culture systems have been utilized for many years and improved upon to address major limitations. Some of the major reasons why the improvement upon these systems has been of interest to ruminant nutritionists is that continuous culture systems are relatively inexpensive to operate and provide a cheaper alternative to test preliminary hypotheses when compared to running an in vivo trial. However, some discrepancies still exist between the accuracy of continuous culture systems and in vivo trials. Prior to the adoption of continuous culture systems batch culture was utilized, but a major pitfall of the batch culture system is they are unable to remove fermentation end products as well as be operated in a stable condition for long periods of time.
Compared to in vivo trials, continuous culture systems are often unable to maintain protozoal populations, although some designs have been modified to improve this [174]. There have also been reports of continuous cultures having major differences in dilution and passage rates, feed input, and lack of the ability to account for absorption parameters [175]. Mansfield et al. [176] investigated the fermentation and microbial ecology of in vivo and in vitro systems and found that concentrations of bacteria were greater, cellulolytic bacteria were also greater, and protozoal populations were greater in vivo when compared to in vitro. Warner [177] was able to maintain a microbial population in vitro for 4 d via the use of a dialysis sac with a buffered solution. However, the microbial population declined rather rapidly due to the accumulation of fermentation end products. Davey et al. [178] observed consistent bacterial numbers in vitro that compared to a 2 week animal experiment comparison. Furthermore, Slyter et al. [179] reported similar fermentation shifts in vivo and in vitro and found that protozoal concentrations decreased from 105 per ml to a level of 2 x 103 per ml after 4 d. In a meta-analysis by Hristov et al. [175] it was found that continuous culture systems typically have lower total VFA and acetate concentration, low or no protozoal populations, and lower OM and NDF digestibility. However, despite these differences continuous culture systems provide an advantage for the quick and safe assessment of experimental treatments. In addition, the utilization of continuous culture systems for studies requiring the analysis of fatty acids in the culture and the effluent provide a great advantage over in vivo systems. Rumen digesta samples are typically simple to obtain and provide a picture of FA metabolism within the rumen. However, FA profiles of rumen fluid may not provide adequate information as to what is available for absorption post-ruminally. Omasal sampling allows researchers to evaluate FA concentration flowing out of the rumen, and thus, available to the animal [179]. However, this process can be difficult and labor intensive. Continuous culture systems possess a reaction vessel that acts as the rumen, and the overflow port where the effluent is removed would be comparative to the omasum in a cow. Sampling of overflow and analyzing for FA composition provide a more accurate representation of what is escaping the rumen and potential milk fat inhibiting isomers that would be available at the intestinal level in the animal. Furthermore, these in vitro systems incubate ground feed with buffer-diluted rumen fluid and are not expected to maintain viability of all microbial populations. Baldin et al. [180] stated that, the microbial populations involved in BH have not been fully characterized, and it is not clear if all key populations are culturable, even in mixed cultures. This is an important consideration in the field of milk fat depression and emphasizes that there is still much more investigation that is warranted.
Amelioration of Milk Fat Depression
Milk fat is one of the most energetically expensive and variable components in milk that is directly altered by nutrition and physiological state [181]. Some nutritional factors that can affect milk fat production include the amount of fiber in the ration, use of ionophores, feeding frequency or pattern, and type of fatty acids in the feed. These factors, combined with other non-nutritional factors such as genetics, stage of lactation, or parity, influence how much milk fat will be produced by the mammary gland. Three theories involved in MFD are the dietary fat deficiency, acetate deficiency, and the glucogenic-insulin theory. However, overall studies provide very little evidence for these theories involving a shortage of precursors for milk fat synthesis as the mechanism of diet-induced MFD. Further, MFD has been shown to occur when acetate is unchanged, thus, disproving the acetate deficiency theory [80]. It is widely accepted that there are a multitude of factors that can contribute to the induction of MFD. McCarthy et al. [182] reported that milk fat trans-10 C18:1 had a negative relationship to herd milk fat percentage as did small TMR particle size. However, the authors were unable to find some univariate relationships with herd-level milk fat. This further suggests the idea that many factors contribute to milk fat content, and herds experiencing low milk fat will need to multiple potential risk factors to solve the problem.
Milk fat depression occurs concordantly with alterations in rumen fermentation as well as the dietary inclusion of PUFA. There are several approaches in order to modify these interactions including diet fermentability, total RUFAL, effective fiber, or the use of rumen modifiers, for example. An example of early work on dietary modification to ameliorate MFD is that of Chalupa et al. [76]. The authors tested if the small amounts of forages in the form of corn silage or baled hay can correct milk fat depression when pellets are provided as the sole source of forage. Not surprisingly, animals receiving pellets continued to have depressed milk fat, but those receiving supplemental forages experienced an increase in milk fat and an increase in de novo FA of milk. The particle size of the diet was thought to be a primary cause for MFD at one point in time, however, we know now that it is more of a factor that contributes to the onset. In addition, work by O’Dell et al. [183] concluded that grind size plays an important role in MFD, and that a grind size of 0.64 cm induces MFD. Grant et al. [184,185] looked at feeding TMR’s with different grind sizes of alfalfa hay or silage and observed a decrease in milk fat for the animals receiving the finely ground treatments. The reasoning behind why milk fat dropped was associated with increased passage rate and decreased digestibility, as well as alterations in VFA [186]. O’Dell et al. [186] reported that increasing feeding frequency to 4 times daily will prevent milk fat depression, but will not recover cows that are already experiencing milk fat depression. Another attempt at recovery was made by Emery et al. [187], who investigated the addition of sodium and potassium bicarbonate addition to cows receiving high-grain rations and found that rumen pH was increased and the bicarbonates prevented a decline in milk fat. Such early works paved the way for common practices that are in place on many dairy farms today and are examples of good management practices overall for a milking herd. After the discovery of the trans-10, cis-12 CLA and confirmation of the biohydrogenation theory, many works focused on identifying other isomers that may reduce milk fat or investigating the mechanism rather than trying to identify other dietary modifications that can be made to ameliorate MFD [72,188,189].
Recent studies have helped to identify the time course of induction and recovery from MFD, and if certain dietary modifications can fix it [2]. Rico et al. [2] were the first to investigate the time course of induction and recovery and found that MFD was induced by 3-5 d, and recovery achieved at 15-19 d. Further work by Rico and others aimed at investigating recovery from milk fat depression by utilizing a variety of techniques [110,190]. In a study by Rico et al. [110] MFD occurs within 7-10 days following a dietary change and 10-14 days is expected to recover milk fat to normal levels after dietary corrections. Methods utilized during recovery phases included modifying diet fermentability by increasing NDF decreasing PUFA concentration. In addition, the authors investigated the changes in ruminal microbial populations during the induction and recovery from MFD and observed that fungi and ciliate protozoa decreased by more than 90% during the induction phase and increased during the recovery phase. Although protozoa do not contribute directly to the biohydrogenation of linoleic acid, they play a major role in starch sequestration and may alter the rumen environment by engulfing large amounts of fermentable starch [191]. Megasphera elsdenii was increased during induction of MFD in the latter study, and has been associated with the production of trans-10, cis-12 CLA [48,191]. Populations of Butyrivibrio have been associated with fatty acid biohydrogenation, and can be inhibited by large amounts of PUFA in the diet [48]. Because rumen microbial populations have been reported to change during MFD, there was also some early work that addressed this with respect to MFD. Satter et al. [192] switched from a high or low forage diet and tested the effect of diet with or without an exchange of rumen contents from cows fed the same diet. They observed that both induction of and recovery from MFD were not only aided by dietary manipulation, but also rumen inoculation from the donor cow. This paved the way for further work by Rico et al. [190], who utilized a similar approach and found that milk fat yield was not altered, but that de novo FA in the milk and rumen FA biohydrogenation was accelerated with inoculation.
Ionophores are commonly fed in dairy cattle diets due to their ability to shift the rumen microbial population to favor more gram-negative bacteria and are important when discussing MFD. Positive effects associated with ionophores include a shift in the acetate:propionate ratio towards more propionate, increased milk production, and reduced risk of acidosis by inhibition of lactic acid production [193]. Current commercially available ionophores include monensin (Rumensin®, lasalocid (Bovatec®), and laidlomycin propionate (Cattlyst®). Their mode of action involves the disruption of ion transfer of Ca2+, Na+, H+, and K+, and may interfere MFD amelioration. However, ionophores may inhibit linoleic acid biohydrogenation by altering the microbial populations responsible for a majority of lipid modification [194]. Several reports have characterized the effects of ionophores, such as monensin, on milk fat yield and concentration [193,195]. There has been some evidence that monensin reduces milk fat yield [195] and others that observed no change [196]. It is evident that the effects of monensin on biohydrogenation and milk fat production are dependent on the nature of the diet. Rico et al. [194] investigated if monensin has an effect on recovery from milk fat depression and found that monensin altered biohydrogenation and milk fat production minimally, but preformed FA in the milk were reduced with monensin. The variable responses with the use of ionophores in diets with high PUFA content suggest that it may be advantageous to exclude them when investigating milk fat depression and recovery.
Despite the often negative connotation of MFD on farm profits, there are instances where controlled MFD may be advantageous. For example, Baumgard [181] noted that milk fat is the most energetically expensive milk component to synthesize and could improve cow health in periods of heat stress or during early lactation. A better understanding of the ruminal transformation and tissue metabolism of fatty acids that potentially alter lipid production provides opportunity to control milk fat at energetically costly periods or physiological stress.
Since MFD is a multifactorial disorder, it is of great importance that we gain a deeper understanding of the nutritional effects on the metabolism of these bioactive fatty acids within the rumen. In some instances, utilization of different nutritional strategies, such as altering the soluble carbohydrate fractions or starch degradability, may help to improve ruminal fermentation and biohydrogenation of linoleic acid. Interactions between nutrient synchronization in the rumen with the use of sugars or neutral detergent soluble fiber with high oil diets are of interest to investigate if MFD can be improved with the addition or subtraction of certain nutrients. Figure 1.5 illustrates some of the many factors involved in milk fat depression and their link to one another. The complexity of the rumen, tissue metabolism, and other factors all contribute to the onset of this disorder.
Conclusion
The complexity of milk fat depression represents a specific challenge for producers. Feeding dairy cows to meet their nutritional requirements is challenged by the needs to meet energy demands without reducing milk and milk fat yield. Factors such as carbohydrate synchronization and microbial protein synthesis, impact on certain microbial populations, and the rumen pH can alter the biohydrogenation of fatty acids and their influence post-rumen. Starch degradability plays an integral role in rumen carbohydrate metabolism and recovery from milk fat depression due to the rate in which starch is fermented can influence microbial populations, rumen pH, and production of milk fat inhibiting isomers. Sugar has shown to be a promising strategy to enhance biohydrogenation and reduce the accumulation of milk fat inhibitors. Modifying other carbohydrate fractions, such as the soluble fiber fraction, is another potential approach to improving conditions within the rumen associated with diet-induced milk fat depression. However, studies investigating nutrient interactions and the effect on omasal outflow are cost-prohibitive in vivo and continuous culture systems provide a similar estimate of what would occur in the animal.
References
- Neville MC, Picciano MF (1997) Regulation of milk lipid secretion and composition. Annu Rev Nutr 17: 159-183.
- Rico DE, Harvatine KJ (2013) Induction of and recovery from milk fat depression occurs progressively in dairy cows switched between diets that differ in fiber and oil concentration. J Dairy Sci 96: 6621-6630.
- Crocker LM, De Peters EJ, Fadel JG, Perez-Monti H, Taylor SJ, et al. (1998) Influence of processed corn grain in diets of dairy cows on digestion of nutrients and milk composition. J Dairy Sci 81: 2394-2407.
- Martel CA, Titgemeyer EC, Mamedova LK, Bradford BJ (2011) Dietary molasses increases ruminal pH and enhances ruminal biohydrogenation during milk fat depression. J Dairy Sci 94: 3995-4004.
- Naderi N, Ghorbani GR, Sadeghi-Sefidmazgi A, Nasrollahi SM, Beauchemin KA, et al. (2016) Shredded beet pulp substituted for corn silage in diets fed to dairy cows under ambient heat stress: Feed intake, total-tract digestibility, plasma metabolites, and milk production. J Dairy Sci 99: 8847-8857.
- Broderick GA, Luchini ND, Reynal SM, Varga GA, Ishler VA, et al. (2008) Effect on production of replacing dietary starch with sucrose in lactating dairy cows. J Dairy Sci 91: 4801-4810.
- Penner GB, Oba M (2009) Increasing dietary sugar concentration may improve dry matter intake, ruminal fermentation, and productivity of dairy cows in the postpartum phase of the transition period. J Dairy Sci 92: 3341-3353.
- Mullins CR, Bradford BJ (2010) Effects of molasses-coated cottonseed product on diet digestibility, performance, and milk fatty acid profile of lactating dairy cattle. J Dairy Sci 93: 3128-3135.
- Voelker JA, Allen MS (2003) Pelleted beet pulp substituted for high-moisture corn: 1. Effects on feed intake chewing behavior, and milk production of lactating dairy cows. J Dairy Sci 86: 3542-3552.
- Münnich M, Khiaosa-ard R, Klevenhusen F, Hilpold A, Khol-Parisini A, et al. (2017) A meta-analysis of feeding sugar beet pulp in dairy cows: Effects on feed intake, ruminal fermentation, performance, and net food production. Anim Feed Sci Technol 224: 78-89.
- Firkins JL, Eastridge ML, St-Pierre NR, Noftsger SM (2001) Effects of grain variability and processing on starch utilization by lactating dairy cattle. J Anim Sci 79: E218-E238.
- Jenkins TC, Fellner V, McGuffey RK (2003) Monensin by fat interactions on trans fatty acids in cultures of mixed ruminal microbes grown in continuous fermenters fed corn or barley. J Dairy Sci 86: 324-330.
- Lascano GJ, Alende M, Koch LE, Jenkins TC (2016) Changes in fermentation and biohydrogenation intermediates in continuous cultures fed low and high levels of fat with increasing rates of starch degradability. J Dairy Sci 99: 6334-6341.
- Azain MJ (2004) Role of fatty acids in adipocyte growth and development. J Anim Sci 82: 916-924.
- Boerman JP, Lock AL (2014) Effect of unsaturated fatty acids and triglycerides from soybeans on milk fat synthesis and biohydrogenation intermediates in dairy cattle. J Dairy Sci 97: 7031-7042.
- Jenkins TC, Harvatine KJ (2014) Lipid feeding and milk fat depression. Vet Clin North Am Food Anim Pract 30: 623-642.
- Van Soest PJ (1994) Nutritional ecology of the ruminant. (2nd edition), Cornell University Press, Ithaca, NY.
- Jenkins TC, McGuire MA (2006) Major advances in nutrition: Impact on milk composition. J Dairy Sci 89: 1302-1310.
- Rico DE, Ying Y, Harvatine KJ (2014) Effect of a high-palmitic acid fat supplement on milk production and apparent total-tract digestibility in high- and low-milk yield dairy cows. J Dairy Sci 97: 3739-3751.
- Palmquist DL, Conrad HR (1980) High fat rations for dairy cows, tallow and hydrolyzed blended fat at two intakes. J Dairy Sci 63: 391-395.
- Jenkins TC, Palmquist DL (1982) Effect of added fat and calcium on in vitro formation of insoluble fatty-acid soaps and cell-wall digestibility. J Anim Sci 55: 957-963.
- Rico DE, Ying Y, Harvatine KJ (2014) Comparison of enriched palmitic acid and calcium salts of palm fatty acids distillate fat supplements on milk production and metabolic profiles of high-producing dairy cows. J Dairy Sci 97: 5637-5644.
- Mohamed OE, Satter LD, Grummer RR, Ehle FR (1988) Influence of dietary cottonseed and soybean on milk production and composition. J Dairy Sci 71: 2677-2688.
- Jenkins TC, Bridges WC (2007) Protection of fatty acids against ruminal biohydrogenation in cattle. Eur J Lipid Sci Technol 109: 778-789.
- Bradford BJ, Allen MS (2004) Milk fat responses to a change in diet fermentability vary by production level in dairy cattle. J Dairy Sci 87: 3800-3807.
- Loften JR, Linn JG, Drackley JK, Jenkins TC, Soderholm CG, et al. (2014) Invited review: Palmitic and stearic acid metabolism in lactating dairy cows. J Dairy Sci 97: 4661-4674.
- Jenkins TC, Palmquist DL (1984) Effect of fatty acids or calcium soaps on rumen and total nutrient digestibility of dairy rations. J Dairy Sci 67: 978-986.
- Schauff DJ, Clark JH (1989) Effects of prilled fatty-acids and calcium salts of fatty-acids on rumen fermentation, nutrient digestibilities, milk production, and milk composition. J Dairy Sci 72: 917-927.
- Enjalbert F, Nicot MC, Bayourthe C, Vernay M, Moncoulon R, et al. (1997) Effects of dietary calcium soaps of unsaturated fatty acids on digestion, milk composition and physical properties of butter. J Dairy Res 64: 181-195.
- Kadegowda AKG, Piperova LS, Delmonte P, Erdman RA (2008) Abomasal infusion of butterfat increases milk fat in lactating dairy cows. J Dairy Sci 91: 2370-2379.
- Livingstone KM, Lovegrove JA, Givens DI (2012) The impact of substituting SFA in dairy products with MUFA or PUFA on CVD ris: Evidence from human intervention studies. Nutr Res Rev 25: 193-206.
- Griinari JM, Corl BA, Lacy SH, Chouinard PY, Nurmela KV, et al. (2000) Conjugated linoleic acid is synthesized endogenously in lactating dairy cows by Delta(9)-desaturase. J Nutr 130: 2285-2291.
- Walstra P, Jenness R (1984) Dairy Chemistry and Physics. John Wiley & Sons, New York, USA.
- Bauman DE, Griinari JM (2003) Nutritional regulation of milk fat synthesis. Annu Rev Nutr 23: 203-227.
- 35Palmquist DL, Conrad HR (1971) Origin of plasma fatty acids in lactating cows fed high grain or high fat diets. J Dairy Sci 54: 1025-1033.
- Moore JH, Christie WW (1979) Lipid metabolism in the mammary gland of ruminant animals. Prog Lipid Res 17: 347-395.
- McGuire JG, Bauman DE (2002) Milk biosynthesis and secretion. In: Encyclopedia of dairy science. Elsevier Science Ltd, London, England, pp: 1828-1834.
- Palmquist DL (2006) Milk fat: Origin of fatty acids and influence of nutritional factors thereon. Advanced Dairy Chemistry, Volume 2: Lipids. (3rd edition), Springer, New York.
- Smith S (1994) The animal fatty acid synthase: One gene, one polypeptide, seven enzymes. Faseb J 8: 1248-1259
- Fox PF, McSweeney PLH (2006) Advanced Dairy Chemistry: Lipids. (3rd edition), Springer, New York.
- Jenkins TC (1993) Lipid Metabolism in the Rumen. J Dairy Sci 76: 3851-3863.
- West CE, Annison EF, Bickerst R, Linzell JL (1972) Studies on mode of uptake of blood triglycerides by mammary gland of lactating goat. The uptake and incorporation into milk fat and mammary lymph of labeled glycerol, fatty-acids and triglycerides. Biochem J 126: 477-490.
- Enoch HG, Catala A, Strittmatter P (1976) Mechanism of Rat-Liver Microsomal Stearyl-Coa Desaturase-Studies of Substrate-Specificity, Enzyme-Substrate Interactions, and Function of Lipid. J Biol Chem 251: 5095-5103.
- Corl BA, Baumgard LH, Dwyer DA, Griinari JM, Phillips BS, et al. (2001) The role of Delta(9)-desaturase in the production of cis-9, trans-11 CLA. J Nutr Biochem 12: 622-630.
- Corl BA, Baumgard LH, Griinari JM, Delmonte P, Morehouse KM, et al. (2002) Trans-7, cis-9 CLA is synthesized endogenously by Delta(9)-desaturase in dairy cows. Lipids 37: 681-688.
- Kinsella JE (1968) Incorporation of [14C3] glycerol into lipids by dispersed bovine mammary cells. Biochim Biophys Acta 164: 540-549.
- Bernard L, Leroux C, Chilliard Y (2008) Expression and nutritional regulation of lipogenic genes in the ruminant lactating mammary gland. Adv Exp Med Biol 606: 67-108.
- Maia MR, Chaudhary LC, Figueres L, Wallace RJ (2007) Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek 91: 303-314.
- 195Davis CL (1990) Fats in Animal Feeds. Barnaby Inc, Sycamore, IL.
- Kairenius P, Leskinen H, Toivonen V, Muetzel S, Ahvenjarvi S, et al. (2018) Effect of dietary fish oil suplements alone or in combination with sunflower and linseed oil on ruminal lipid metabolism and bacteria populations in lactating cows. J Dairy Sci 101: 3021-3035.
- Chilliard Y, Glasser F, Ferlay A, Bernard L, Rouel J, et al. (2007) Diet, rumen biohydrogenation and nutritional quality of cow and goat milk fat. Europ J Lipid Sci Techn 109: 828-855.
- Harfoot CG, Hazelwood GP (1988) Lipid metabolism in the rumen. In: The Rumen Microbial Ecosystem. Elsevier Applied Science Publishers, London, pp: 285-322.
- Latham MJ, Storry JE, Sharpe ME (1972) Effect of lowroughage diets on the microflora and lipid metabolism in the rumen. Appl Microbiol 24: 871-877.
- Weimer PJ, Stevenson DM, Mertens DR (2010) Shifts in bacterial community composition in the rumen of lactating dairy cows under milk fat-depressing conditions. J Dairy Sci 93: 265-278.
- Weimer PJ, Da Silva Cabral L, Cacite F (2015) Effects of ruminal dosing of Holstein cows with Megasphaera elsdenii on milk fat production, ruminal chemistry, and bacterial strain persistence. J Dairy Sci 98: 8078-8092.
- Boeckaert C, Vlaeminck B, Fievez V, Maignien L, Dijkstra J, et al. (2008) Accumulation of trans-C18:1 fatty acids in the rumen after dietary algal supplementation is associated with changes in the Butyrivibrio community. Appl Environ Microbiol 74: 6923-6930.
- Kepler CR, Hiron KP, McNeill JJ, Tove SB (1966) Intermediates and products of the biohydrogenation of linoleic acid by Butyrivibrio fibrisolvens. J Biol Chem 241: 1350-1354.
- Verhulst A, Janssen G, Parmentier G, Eyssen H (1987) Isomerization of polyunsaturated fatty acids by Propionibacteria. Sys Appl Microbiol 9: 12-15.
- Kim YJ, Liu RH, Bond D, Russell JB (2000) The effect of linoleic acid concentration on the conjugated linoleic acid (CLA) production of Butyrivibrio fibrisolvens A38. Appl Enviro Microbiol 66: 5226-5230.
- Keeney M, (1970) Lipid metabolism in the rumen. Physiology of Digestion and Metabolism in the Ruminant. Oriel Press Limited, Newcastle upon Tyne, UK, pp: 489-503.
- Chalupa W, O'Dell GD, Kutches AJ, Lavker R (1967) Changes in rumen chemical characteristics and protozoa populations of animals with depressed milk fat tests. J Dairy Sci 50: 1002.
- Chalupa W, Kutches AJ (1970) Biohydrogenation of linoleic- 1-14C-acid by rumen protozoa. J Anim Sci 27: 1502-1508.
- Devillard E, Wallace RJ (2006) Biohydrogenation in the rumen and human intestine: Implications for CLA and PUFA. Lipid Technol 18: 127-130.
- Orpin CG (1984) The role of ciliate protozoa and fungi in the rumen digestion of plant-cell walls. Anim Feed Sci Technol 10: 121-143.
- Nam IS, Garnsworthy PC (2007) Factors influencing biohydrogenation and conjugated linoleic acid production by mixed rumen fungi. J Microbiol 45: 199-204.
- Bauman DE, Perfield JW, Harvatine KJ, Baumgard LH (2008) Regulation of fat synthesis by conjugated linoleic acid: Lactation and the ruminant model. J Nutr 138: 403-409.
- Lee YJ, Jenkins TC (2011) Identification of enriched conjugated linoleic acid isomers in cultures of ruminal microoganisms after dosing with 1-13C-linoleic acid. J Micro 49: 622-627.
- Harvatine KJ, Boisclair YR, Bauman DE (2009) Recent advances in the regulation of milk fat synthesis. Animal 3: 40-54.
- Baumgard LH, Corl BA, Dwyer DA, Saebo A, Bauman DE, et al. (2000) Identification of the conjugated linoleic acid isomer that inhibits milk fat synthesis. Am J Physiol Regul Integr Comp Physiol 278: R179-R184.
- Saebo A, Saebo PC, Griinari JM, Shingfield KJ (2005) Effect of abomasal infusions of geometric isomers of 10,12 conjugated synthesis linoleic acid on milk fat in dairy cows. Lipids 40: 823-832.
- Shingfield KJ, Reynolds CK, Lupoli B, Toivonen V, Yurawecz MP, et al. (2005) Effect of forage type and proportion of concentrate in the diet on milk fatty acid composition in cows given sunflower oil and fish oil. Anim Sci 80: 225-238.
- Perfield JW, Lock AL, Griinari JM, Saebo A, Delmonte P, et al. (2007) Trans-9, cis-11 conjugated linoleic acid reduces milk fat synthesis in lactating dairy cows. J Dairy Sci 90: 2211-2218.
- Nogalski Z, Wronski M, Sobczuk-Szul M, Mochol M, Pogorzelska P, et al. (2012) The effect of body energy reserve mobilization on the fatty acid profile of milk in high-yielding cows. Asian-Australas J Anim Sci 25: 1712-1720.
- Jorgensen NA, Barr GR, Schultz LH (1964) Factors influencing milk fat depression on restricted roughage diets. J Dairy Sci 47: 688.
- Jorgensen NA, Schultz LH, Barr GR (1965) Factors influencing milk fat depression on rations high in concentrates. J Dairy Sci 48: 1031-1039.
- Chalupa W, Odell GD, Kutches AJ, Lavker R (1970) Supplemental corn silage or baled hay for correction of milk fat depression produced by feeding pellets as sole forage. J Dairy Sci 53: 208-214.
- Drummond JC, Channon HJ, Coward KH, Golding J, Mackintosh J, et al. (1924) The influence of the administration of certain oils on the nutritive value of the butter fat of cows on winter rations. J Agr Sci 14: 531-547.
- Dann WJ, Moore T (1935) A new spectroscopic phenomenon in fatty acid metabolism. The conversion of "pro-absorptive" to "absorptive" acids in the cow. Biochem J 29: 138-146.
- Balch CC, Balch DA, Bartlett S, Cox CP, Rowland SJ, et al. (1952) Studies of the secretion of milk of low fat content by cows on diets low in hay and high in concentrates. I. The effect of variations in the amount of hay. J Dairy Res 19: 39-50.
- Davis CL, Brown RE (1970) Low-fat milk syndrome. Physiology of Digestion and Metabolism in the Ruminant, Newcastle-upon-Tyne: Oriel Press, UK, pp: 545-565.
- Tyznik W, Allen NN (1951) The relation of roughage intake to the fat content of the milk and the level of fatty acids in the rumen. J Dairy Sci 34: 493-493.
- McClymont GL (1950) The relation of the type and quantity of roughage and grazing to the fat content of milk. Australian Vet J 6: 111.
- Mcclymont GL, Vallance S (1962) Depression of blood glycerides and milk-fat synthesis by glucose infusion. P Nutr Soc 21: R41.
- Frobish RA, Davis CL (1977) Theory involving propionate and vitamin-B12 in low-milk fat syndrome. J Dairy Sci 60: 268-273.
- Mcguire MA, Griinari JM, Dwyer DA, Bauman DE (1995) Role of insulin in the regulation of mammary synthesis of fat and protein. J Dairy Sci 78: 816-824.
- Bauman DE, Griinari JM (2001) Regulation and nutritional manipulation of milk fat: low-fat milk syndrome. Livest Prod Sci 70: 15-29.
- Corl BA, Butler ST, Butler WR, Bauman DE (2006) Short communication: regulation of milk fat yield and fatty acid composition by insulin. J Dairy Sci 89: 4172-4175.
- Mackle TR, Dwyer DA, Ingvartsen KL, Chouinard PY, Lynch JM, et al. (1999) Effects of insulin and amino acids on milk protein concentration and yield from dairy cows. J Dairy Sci 82: 1512-1524.
- Griinari JM, McGuire MA, Dwyer DA, Bauman DE, Palmquist DL, et al. (1997) Role of insulin in the regulation of milk fat synthesis in dairy cows. J Dairy Sci 80: 1076-1084.
- Walker CK, Elliot JM (1972) Lactational trends in vitamin B12 status on conventional and restricted roughage rations. J Dairy Sci 55: 474-479.
- Elliot JM, Barton EP, Williams JA (1979) Milk fat as related to vitamin B12 status. J Dairy Sci 62: 642-645.
- Croom WJ, Rakes AH, Linnerud AC, Ducharne GA, Elliott JM, et al. (1981) Vitamin B12 administration for milk fat synthesis in lactating dairy cows fed a low fiber diet. J Dairy Sci 61: 1555-1560.
- Gaynor PJ, Erdman RA, Teter BB, Sampugna J, Capuco AV, et al. (1994) Milk fat yield and composition during abomasal infusion of cis or trans octadecenoates in Holstein cows. J Dairy Sci 77: 157-65 57.
- Gaynor PJ, Waldo DR, Capuco AV, Erdman RA, Douglass LW, et al. (1995) Milk fat depression, the glucogenic theory, and trans-C18:1 fatty acids. J Dairy Sci 78: 2008-2015.
- Erdman R (1999) Trans fatty acids and fat synthesis in milk. Proc Southwest Nutr Mgt Conf, Univ. Arizona, Tucson, pp: 113-25..
- Kalscheur KF, Teter BB, Piperova LS, Erdman RA (1997) Effect of fat source on duodenal flow of trans-C18:1 fatty acids and milk fat production in dairy cows. J Dairy Sci 80: 2115-2126.
- Selner DR, Schultz LH (1980) Effects of feeding oleic acid or hydrogenated vegetable oils to lactating cows. J Dairy Sci 63: 1235-1241.
- Griinari JM, Dwyer DA, McGuire MA, Bauman DE, Palmquist DL, et al. (1998) Trans-octadecenoic acids and milk fat depression in lactating dairy cows. J Dairy Sci 81: 1251-1261.
- Griinari JM, Bauman DE (1999) Biosynthesis of conjugated linoleic acid and its incorporation into meat and milk in ruminants. In Advances in Conjugated Linoleic Acid Research, Champaign, IL: AOCS Press, USA, pp: 180-200.
- Fuentes MC, Calsamiglia S, Cardozo PW, Vlaeminck B (2009) Effect of pH and level of concentrate in the diet on the production of biohydrogenation intermediates in dual-flow continuous culture. J Dairy Sci 92: 4456-4466.
- Pariza MW, Ashoor SH, Chu FS, Lund DB (1979) Effects of temperature and time on mutagen formation in pan-fried hamburger. Cancer Lett 7: 63-69.
- Pariza MW, Hargraves WA (1985) A beef-derived mutagenesis modulator inhibits initiation of mouse epidermal tumors by 7,12-dimethylbenz[a]anthracene. Carcinogenesis 6: 591-593.
- Pariza MW, Loretz LJ, Storkson JM, Holland NC (1983) Mutagens and modulator of mutagenesis in fried ground-beef. Cancer Res 43: 2444-2446.
- Chouinard PY, Corneau L, Barbano DM, Metzger LE, Bauman DE, et al. (1999) Conjugated linoleic acids alter milk fatty acid composition and inhibit milk fat secretion in dairy cows. J Nutr 129: 1579-1584.
- Baumgard LH, Sangster JK, Bauman DE (2001) Milk fat synthesis in dairy cows is progressively reduced by increasing supplemental amounts of trans-10, cis-12 conjugated linoleic acid (CLA). J Nutr 131: 1764-1769.
- Peterson DG, Baumgard LH, Bauman DE (2002) Short communication: Milk fat response to low doses of trans-10, cis-12 conjugated linoleic acid (CLA). J Dairy Sci 85: 1764-1766.
- Loor JJ, Herbein JH (2003) Reduced fatty acid sunthesis and desaturation due to exogenous trans-10, cis-12 CLA in cows fed oleic or linoleic oil. J Dairy Sci 86: 1354-1369.
- Jenkins TC, Bridges WC, Harrison JH, Young KM (2014) Addition of potassium carbonate to continuous cultures of mixed ruminal bacteria shifts volatile fatty acids and daily production of biohydrogenation intermediates. J Dairy Sci 97: 975-984.
- Harvatine KJ, Allen MS (2006) Effects of fatty acid supplements on milk yield and energy balance of lactating dairy cows. J Dairy Sci 89: 1081-1091.
- Rico DE, Holloway AW, Harvatine KJ (2015) Effect of diet fermentability and unsaturated fatty acid concentration on recovery from diet-induced milk fat depression. J Dairy Sci 98: 7930-7943.
- Bionaz M, Loor JJ (2008) Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics 9: 366.
- Hussein M, Harvatine KH, Weerasinghe WPMB, Sinclair LA, Bauman DE, et al. (2013) Conjugated linoleic acid-induced milk fat depression in lactating ewes is accompanied by reduced expression of mammary genes involved in lipid synthesis. J Dairy Sci 96: 3825-3834.
- Szymczyk B, Pisulewski PM, Szczurek W, Hanczakowski P (2001) Effects of conjugated linoleic acid on growth performance, feed conversion efficiency, and subsequent carcass quality in broiler chickens. Br J Nutr 85: 465-473.
- Corino C, Mourot J, Magni S, Pastorelli G, Rosi F, et al. (2002) Influence of dietary conjugated linoleic acid on growth, meat quality, lipogenesis, plasma leptin and physiological variables of lipid metabolism in rabbits. J Anim Sci 80: 1020-1028.
- Hausman GJ, Dodson MV, Ajuwon K, Azain M, Barnes KM, et al. (2009) Board-invited review: The biology and regulation of preadipocytes and adipocytes in meat animals. J Anim Sci 87: 1218-1246.
- Loor JJ, Lin XB, Herbein JH (2003) Effects of dietary cis 9, trans 11-18:2, trans 10, cis 12-18:2 or vaccenic acid (trans 11-18:1) during lactation on body composition, tissue fatty acid profiles, and litter growth in mice. Br J Nutr 90: 1039-1048.
- Bontempo V, Sciannimanico D, Pastorelli G, Rossi R, Rosi F, et al. (2004) Dietary conjugated linoleic acid positively affects immunologic variables in lactating sows and piglets. J Nutr 134: 817-824.
- Lock AL, Teles BM, Perfield JW, Bauman DE, Sinclair LA, et al. (2006) A conjugated linoleic acid supplement containing trans-10, cis-12 reduces milk fat synthesis in lactating sheep. J Dairy Sci 89: 1525-1532.
- Muller M, Kersten S (2003) Nutrigenomics: Goals and strategies. Nat Rev Genet 4: 315-322.
- Ahnadi CE, Beswick N, Delbecchi L, Kennelly JJ, Lacasse P, et al. (2002) Addition of fish oil to diets for dairy cows. II. Effects on milk fat and gene expression of mammary lipogenic enzymes. J Dairy Res 69: 521-531.
- Zhang TY, Huang JT, Tian HB, Ma Y, Chen Z, et al. (2018) Trans-10, cis-12 conjugated linoleic acid alters lipid metabolism of goat mammary epithelial cells by regulation of de novo synthesis and the AMPK signaling pathway. J Dairy Sci 101: 1-11.
- Harvatine KJ, Bauman DE (2006) SREBP1 and thyroid hormone responsive spot 14 (S14) are involved in the regulation of bovine mammary lipid synthesis during diet-induced milk fat depression and treatment with CLA. J Nutr 136: 2468-2474.
- Vyas D, Moallem U, Teter BB, Fardin-Kia ARK, Erdman RA, et al. (2013) Milk fat responses to butterfat infusion during conjugated linoleic acid-induced milk fat depression in dairy cows. J Dairy Sci 96: 2387-2399.
- Peterson, DG, Matitashvili EA, Bauman DE (2004) The inhibitory effect of trans-10, cis-12 CLA on lipid synthesis in bovine mammary epithelial cells involves reduced proteolytic activation of the transcription factor SREBP-1. J Nutr 134: 2523-2527.
- Harvatine KJ, Perfield II JW, Bauman DE (2009) Expression of enzymes and key regulators of lipid synthesis is upregulated in adipose tissue during CLA-induced milk fat depression in dairy cows. J Nutr 139: 821-825.
- NRC (2001) Nutrient Requirements of Dairy Cattle, (7th edition), National Acad Press, Washington, DC.
- Rosser R, Polan C, Chandler P, Bibb T (1971) Effects of whey components and methionine analog on bovine milk fat production. J Dairy Sci 54: 1807-1816.
- Huber TJ, Emery R, Bergen W, Liesman J, Kung L, et al. (1984) Influences of methionine hydroxy analog on milk and milk fat production, blood serum lipids, and plasma amino acids. J Dairy Sci 67: 2525-2531.
- Baldin M, Zanton GI, Harvatine KJ (2018) Effect of 2-hydroxy-4-(methylthio)butanoate (HMTBa) on risk of biohydrogentation-induced milk fat depression. J Dairy Sci 101: 376-385.
- Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74: 3583-3597.
- Strobel HJ, Russell JB (1986) Effect of pH and energy spilling on bacterial protein-synthesis by carbohydrate limited cultures of mixed rumen bacteria. J Dairy Sci 69: 2941-2947.
- Leiva E, Hall MB, Van Horn HH (2000) Performance of dairy cattle fed citrus pulp or corn products as sources of neutral detergent-soluble carbohydrates. J Dairy Sci 83: 2866-2875.
- Dann HM, Carter MP, Cotanch KW, Ballard CS, Takano T, et al. (2007) Effect of partial replacement of forage neutral detergent fiber with by-product neutral detergent fiber in close-up diets on periparturient performance of dairy cows. J Dairy Sci 90: 1789-1801.
- Ferraretto LF, Crump PM, Shaver RD (2013) Effect of cereal grain type and corn grain harvesting and processing methods on intake, digestion, and milk production by dairy cows through a meta-analysis. J Dairy Sci 96: 533-550.
- Hall MB, Herejk C (2001) Differences in yields of microbial crude protein from in vitro fermentation of carbohydrates. J Dairy Sci 84: 2486-2493.
- Ariza P, Bach A, Stern MD, Hall MB (2001) Effects of carbohydrates from citrus pulp and hominy feed on microbial fermentation in continuous culture. J Anim Sci 79: 2713-2718.
- Santos-Silva J, Dentinho MT, Francisco A, Portugal AP, Belo AT, et al. (2016) Replacing cereals with dehydrated citrus pulp in a soybean oil supplemented diet increases vaccenic and rumenic acids in ewe milk. J Dairy Sci 99: 1173-1182.
- Santos FAP, Menezes Júnior MP, Simas JMC, Pires AV, Nussio CMB, et al. (2001) Corn grain processing and its partial replacement by pelleted citrus pulp on performance, nutrient digestibility and blood parameters of dairy cows. Acta Sci Anim Sci 23: 923-931.
- de Assis AJ, de Souza Campos JM, Valadares SD, de Queiroz AC, Lana RD, et al. (2004) Citrus pulp in diets for milking cows. Intake of nutrients, milk production and composition. R Bras Zootec 33: 242-250.
- Bradford BJ, Mullins CR (2012) Invited review: Strategies for promoting productivity and health of dairy cattle by feeding nonforage fiber sources. J Dairy Sci 95: 4735-4746.
- Solomon R, Chase LE, Ben-Ghedalia D, Bauman DE (2000) The effect of nonstructural carbohydrate and addition of full fat extruded soybeans on the concentration of conjugated linoleic acid in the milk of dairy cows. J Dairy Sci 83: 1322-1329.
- Mansfield HR, Stern MD, Otterby DE (1994) Effects of beet pulp and animal by-products on milk-yield and in-vitro fermentation by rumen microorganisms. J Dairy Sci 77: 205-216.
- Van Knegsel A, Remmelink G, Jorjong S, Fievez V, Kemp B, et al. (2014) Effect of dry period length and dietary energy source on energy balance, milk yield, and milk composition of dairy cows. J Dairy Sci 97: 1499-1512.
- Shahmoradi A, Alikhani M, Riasi A, Ghorbani G, Ghaffari M, et al. (2015) Effects of partial replacement of barley grain with beet pulp on performance, ruminal fermentation and plasma concentration of metabolites in transition dairy cows. J Anim Physiol Anim Nutr 100: 178-188.
- Boguhn J, Kluth H, Bulang M, Engelhard T, Rodehutscord M, et al. (2010) Effects of pressed beet pulp silage inclusion in maize-based rations on performance of high-yielding dairy cows and parameters of rumen fermentation. Animal 4: 30-39.
- Heldt JS, Cochran RC, Stokka GK, Farmer CG, Mathis CP, et al. (1999) Effects of different suppplemental sugars and starch fed in combination with degradable intake protein on low-quality forage use by beef steers. J Anim Sci 77: 2793-2802.
- Kellogg DW (1969) Influence of sucrose on rumen fermentation pattern and milk fat content in cows fed a high-grain ration. J Dairy Sci 52: 657-662.
- Ribeiro CVDM, Karnati SKR, Eastridge ML (2005) Biohydrogenation of fatty acids and digestibility of fresh alfalfa or alfalfa hay plus sucrose in continuous culture. J Dairy Sci 88: 4007-4017.
- Harvatine DI, Firkins JL, Eastridge ML (2000) Whole linted cottonseed as a forage substitute fed with ground or steam-flaked corn: Digestibility and performance. J Dairy Sci 85: 1976-1987.
- Theurer CB, Huber JT, Delgado-Elorduy A, Wanderley R (1999) Invited review: Summary of steam-flaking or sorghum grain for lactating dairy cows. J Dairy Sci 82: 1950-1959.
- Dann HM, Varga GA, Putnam DE (1999) Improving energy supply to late gestation and early postpartum dairy cows. J Dairy Sci 82: 1765-1778.
- Broderick GA, Radloff WG (2004) Effect of molasses supplementation on the production of lactating dairy cows fed diets based on alfalfa and corn silage. J Dairy Sci 87: 2997-3009.
- Penner GB, Beauchemin KA, Mutsvangwa T (2007) Severity of ruminal acidosis in primiparous Holstein cows during the periparturient period. J Dairy Sci 90: 365-375.
- Firkins J (2010) Addition of sugars to dairy rations. Tri-State Dairy Nutrition Conference. The Ohio State University, Columbus, pp: 91-105.
- Oba M (2011) Review: Effects of feeding sugars on productivity of lactating dairy cows. Canadian J Anim Sci 91: 37-46.
- Guan LL, Nkrumah JD, Basarab JA, Moore SS (2008) Linkage of microbial ecology to phenotype: correlation of rumen microbial ecology to cattle's feed efficiency. Fems Microbiol Lett 288: 85-91.
- Vallimont JE, Bargo F, Cassidy TW, Luchini ND, Broderick GA, et al. (2004) Effects of replacing dietary starch with sucrose on ruminal fermentation and nitrogen metabolism in continuous culture. J Dairy Sci 87: 4221-4229.
- Oba M, Lewis JL, Zhining Z (2015) Effects of ruminal doses of sucrose, lactose, and corn starch on ruminal fermentation and expression of genes in ruminal epithelial cells. J Dairy Sci 98: 586-594.
- Weisbjerg MR, Hvelplund T, Bibby BM (1998) Hydrolysis and fermentation rate of glucose, sucrose and lactose in the rumen. Acta Agric Scand A Anim Sci 48: 12-18.
- Sutton JD (1968) The fermentation of soluble carbohydrates in rumen contents of cows fed diets containing a large proportion of hay. Br J Nutr 22: 689-712.
- Chamberlain DG, Robertson S, Choung JJ (1993) Sugars versus starch as supplements to grass silage: Effects on ruminal fermentation and the supply of microbial protein to the small intestine, estimated from the urinary excretion of purine derivatives, in sheep. J Sci Food Agric 63: 189-194.
- Sano H, Hayakawa S, Takahashi H, Terashima Y (1995) Plasma insulin and glucagon responses to propionate infusion into femoral and mesenteric veins in sheep. J Anim Sci 73: 191-197.
- Chibisa GE, Gorka P, Penner GB, Berthiaume R, Mutsvangwa T, et al. (2015) Effects of partial replacement of dietary starch from barley or corn with lactose on ruminal function, short-chain fatty acid absorption, nitrogen utilization, and production performance of dairy cows. J Dairy Sci 98: 2627-2640.
- Sun X, Wang Y, Chen B, Zhao X (2015) Partially replacing cornstarch in a high-concentrate diet with sucrose inhibited the ruminal trans-10 biohydrogenation pathway in vitro by changing populations of specific bacteria. J Anim Sci Biotechnol 6: 57.
- Nombekela SW, Murphy MR (1995) Sucrose supplementation and feed-intake of dairy-cows in early lactation. J Dairy Sci 78: 880-885.
- Shingfield KJ, Griinari JM (2007) Role of biohydrogenation intermediates in milk fat depression. Eur J Lipid Sci Tech 109: 799-816.
- Razzaghi A, Valizadeh R, Naserian AA, Danesh Mesgaran M, Carpenter AJ, et al. (2016) Effect of dietary sugar concentration and sunflower seed supplementation on lactation performance, ruminal fermentation, milk fatty acid profile, and blood metabolites of dairy cows. J Dairy Sci 99: 3539-3548.
- Dann HM, Fredin SM, Cotanch KW, Grant RJ, Kokko C, et al. (2015) Effects of corn-based reduced-starch diets using alternative carbohydrate sources on performance of lactating Holstein cows. J Dairy Sci 98: 4041-4054.
- Chen KH, Huber JT, Theurer CB, Swingle RS, Simas J, et al. (1994) Effect of steam flaking of corn and sorghum grains on performance of lactating cows. J Dairy Sci 77: 1038-1043.
- Lechartier C, Peyraud JL (2010) The effects of forage proportion and rapidly degradable dry matter from concentrate on ruminal digestion in dairy cows fed corn silage based diets with fixed neutral detergent fiber and starch contents. J Dairy Sci 93: 666-681.
- Peyrat J, Baumont R, Le Morvan A, Nozière P (2016) Effect of maturity and hybrid on ruminal and intestinal digestion of corn silage in dry cows. J Dairy Sci 99: 258-268.
- Hatew B, Podesta SC, Van Laar H, Pellikaan WF, Ellis JL, et al. (2015) Effects of dietary starch content and rate of fermentation on methane production in lactating dairy cows. J Dairy Sci 98: 486-499.
- Huntington GB (1997) Starch utilization by ruminants: From basics to the bunk. J Anim Sci 75: 852-867.
- Teather RM, Sauer FD (1988) A naturally compartmented rumen simulation system for the continuous culture of rumen bacteria and protozoa. J Dairy Sci 71: 666-673.
- Hristov AN, Lee C, Hristova R, Huhtanen P, Firkins JL, et al. (2012) A meta-analysis of variability in continuous culture ruminal fermentation and digestibility data. J Dairy Sci 95: 5299-5307.
- Mansfield HR, Endres MI, Stern MD (1995) Comparison of microbial fermentation in the rumen of dairy cows and dual flow continuous culture. Anim Feed Sci Technol 55: 47-66.
- Warner ACI (1956) Criteria for establishing the validity of in vitro studies with rumen microorganisms in so-called artificial rumen systems. J Gen Microbiol 14: 733-748.
- Davey LA, Chesseman GC, Briggs CAE (1960) Evaluation of an improved artificial rumen designed for continuous control during prolonged operation. J Agr Sci 55: 155-163.
- Slyter LL, Nelson WO, Wolin MJ (1964) Modifications of a device for maintenance of the rumen microbial population in continuous culture. Appl Microbiol 12: 374-377.
- Baldin M, Rico DE, Green MH, Harvatine KJ (2018) Technical note: An in vivo method to determine kinetics of unsaturated fatty acid biohydrogenation in the rumen. J Dairy Sci 101: 1-9.
- Baumgard LH (2002) Managing milk fat depression. University of Arizona Producers Conference, pp: 41-46.
- McCarthy MM, Overton TR, Mechor GD, Bauman DE, Jenkins TC, et al. (2018) Short communication: Field study to investigate the associations between herd-level risk factors for milk fat depression and mulk tank milk fat percent in dairy herds feeding monensin. J Dairy Sci 101: 3118-3125.
- O'Dell GD, King WA, Cook WC (1968) Effect of grinding, pelleting, and frequency of feeding forage on fat percentage of milk and milk production of dairy cows. J Dairy Sci 51: 50-55.
- Grant RJ, Colenbrander VF, Mertens DR (1990) Milk fat depression in dairy cows: Role of particle size of alfalfa hay. J Dairy Sci 73: 1823-1833.
- Grant RJ, Colenbrander VF, Mertens DR (1990) Milk fat depression in dairy cows: Role of silage particle size. J Dairy Sci 73: 1834-1842.
- O'Dell GD, King WA, Cook WC, Balk WA (1964) Effects of forage supplementation and frequency of feeding of pelleted Coastal Bermudagrass hay on milk and milk fat production. J Dairy Sci 47: 648.
- Emery RS, Brown LD (1961) Effect of feeding sodium and potassium bicarbonate on milk fat, rumen pH, and volatile fatty acid production. J Dairy Sci 44: 1899-1902.
- Perfield II JW, Saebø A, Bauman DE (2004) Use of conjugated linoleic acid (CLA) enrichments to examine the effects of trans-8, cis-10 CLA, and cis-11, trans-13 CLA on milk-fat synthesis. J Dairy Sci 87: 1196-1202.
- Perfield II JW, Delmonte P, Lock AL, Yurawecz MP, Bauman DE, et al. (2006) Trans-10, trans-12 conjugated linoleic acid does not affect milk fat yield but reduces Δ9 -desaturase index in dairy cows. J Dairy Sci 89: 2559-2566.
- Rico DE, Ying Y, Clarke AR, Harvatine KJ (2014) The effect of rumen digesta inoculation on the time course of recovery from classical diet-induced milk fat depression in dairy cows. J Dairy Sci 97: 3752-3760.
- Rico DE, Preston SH, Risser JM, Harvatine KJ (2015) Rapid changes in key ruminal microbial populations during the induction of and recovery from diet-induced milk fat depression in dairy cows. Br J Nutr 114: 358-367.
- Satter LD, Bringe AN (1969) Effect of abrupt ration changes on milk and blood components. J Dairy Sci 52: 1776-1780.
- Odongo NE, Bagg R, Vessie G, Dick P, Or-Rashid MM, et al. (2007) Long-term effects of feeding monensin on methane production in lactating dairy cows. J Dairy Sci 90: 2577-2577.
- Rico DE, Holloway AW, Harvatine KJ (2014) Effect of monensin on recovery from diet-induced milk fat depression. J Dairy Sci 97: 2376-2386.
- Phipps RH, Wilkinson JID, Jonker LJ, Tarrant M, Jones AK, et al. (2000) Effect of monensin on milk production of Holstein-Friesian dairy cows. J Dairy Sci 83: 2789-2794.
- He M, Perfield KL, Green HB, Armentano LE (2012) Effect of dietary fat blend enriched in oleic or linoleic acid and monensin supplementation on dairy cattle performance, milk fatty acid profiles, and milk fat depression. J Dairy Sci 95: 1447-1461.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences