Effect of Selenium Nanoparticles on Anti-Oxidative Level, Egg Production and Quality and Blood Parameter of Laying Hens Exposed to Deoxynivalenol
Wenhui Qu, Junhua Yang, Zhenzhen Sun, Ruihua Zhang, Fei Zhou, Kechun Zhang, Ye Xia, Kehe Huang, Denian Miao
DOI10.21767/2572-5459.100021
Wenhui Qu1,2, Junhua Yang2, Zhenzhen Sun3, Ruihua Zhang2, Fei Zhou2, Kechun Zhang1,4, Ye Xia3, Kehe Huang1 and Denian Miao1,3*
1College of Veterinary Medicine, Nanjing Agricultural University, Nanjing, Jiangsu Province, China
2Shanghai Institute of Dairy Science, Shanghai, China
3Shanghai Academy of Agricultural Sciences, Shanghai, China
4Nanjing Agricultural University, Nanjing, Jiangsu Province, China
- Corresponding Author:
- Miao D
Institute of Animal Husbandry and Veterinary Science
Shanghai Academy of Agricultural Sciences (SAAS), Shanghai, PR China
Tel: +86-21-62202519
Fax: +86-21-62207858
E-mail: mdn2008@gmail.com
Received Date: January 10, 2017; Accepted Date: January 25, 2017; Published Date: January 27, 2017
Citation: Qu W, Yang J, Sun Z, Zhang R, Zhou F, et al. (2017) Effect of Selenium Nanoparticles on Anti-Oxidative Level, Egg Production and Quality and Blood Parameter of Laying Hens Exposed to Deoxynivalenol. J Anim Res Nutr. 2:1. doi: 10.21767/2572-5459.100021
Copyright: © 2017 Qu W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The aim of this study was to investigate the efficacy of selenium nanoparticles (SeNPs) that had been biologically synthesized by Bacillus licheniformis to counteract deoxynivalenol (DON) toxicity in laying hens. Ninety-six healthy, 20-week-old laying hens were randomly assigned to four treatment groups, each of which included 3 replicates of 8 layers, and fed four different diets: an uncontaminated basal diet (control group), a 10 mg/kg DON-contaminated basal diet (DON group), a 10 mg/kg DON-contaminated basal diet with 0.5 mg/kg SeNPs (DON+SeNPs group) and the basal diet with 0.5 mg/kg SeNPs (SeNPs group).
Serum T-AOC and GPx activities were significantly decreased (P<0.05) in hens fed the DON-contaminated diet, and DON significantly decreased the egg production rate (P<0.05), significantly increased the soft-shelled or cracked egg rate (P<0.05), significantly decreased serum calcium and inhibited the immune systems of the animals according to blood routine indexes. However, SeNPs improved the levels of GPx and T-AOC, increased the egg production rate, significantly decreased the soft-shelled or cracked egg rate (P<0.05), and decreased the influence of DON on the blood routine.
In addition, SeNPs significantly (P<0.05) increased serum calcium. However, no differences were observed in egg quality (egg weight, Haugh units, yolk color, eggshell strength and eggshell thickness) among the four groups. These results showed that SeNPs could provide effective anti-oxidative protection against DON toxicity in laying hens, reduced DON’s influence on egg production and blood calcium
Keywords
Deoxynivalenol (DON); Selenium nanoparticles (SeNPs); Antioxidation; Laying hens
Introduction
Deoxynivalenol (DON), also known as vomitoxin, is one of several mycotoxins produced by certain Fusarium species that frequently infect corn, wheat, and other grains in the field or during storage, resulting in great economic losses [1]. To date, many studies have suggested that the presence of DON in food and feed negatively affects health and productivity, particularly by causing anorexia and vomiting [2,3], but different species exhibit different sensitivities. Although laying hens are not as sensitive to DON as some other species [4], the toxin does pose a major threat. The productive and growth performance of laying hens, as well as the related economic income, seriously decrease as the DON concentration increases. Even at a low concentration, DON can cause serious damage at the cellular level.
Previous studies of the mechanisms underlying DON toxicity have mainly focused on absorption, metabolism and the immune response [5], but recent studies have paid more attention to DON-induced oxidative stress. DON could increase the production of free radicals, thus initiating oxidative stress [6-9], and both in vitro [10-12] and in vivo [13-15] studies have suggested that DON causes DNA fragmentation and lipid peroxidation (LPO), which lead to cell death and apoptosis through oxidative stress. On the other hand, previous research has found that antioxidants including epigallocatechin gallate (EGCG) [16], lutin [17], flavanols and vitamins E (α-tocopherol), A (β-carotene) or C (ascorbic acid) [18] could protect animals from DON toxicity. All of the above studies demonstrate the key role of oxidative stress in DON toxicity from different perspectives.
Selenium (Se) is an essential trace element for animals and humans that is commonly used as an antioxidant, but there is a very narrow margin between the nutrient levels that are safe for intake and those that are toxic [19]. Selenium nanoparticles (SeNPs), a new source of selenium, exhibit the excellent biological properties of antioxidant and antibacterial activities as well as lower toxicity, better absorption and bioactivity.
Especially, some reports reported selenium nanoparticles have anticancer effect. Wang et al. [20] found Se-HANs induced apoptosis of tumor cells by an inherent caspase-dependent apoptosis pathway synergistically orchestrated with the generation of reactive oxygen species. In addition, another research reported that selenite-doped bone mineral nanoparticles can retard the growth of osteosarcoma in a nude mice model [21]. These excellent properties introduced selenium nanoparticles as a good candidate for replacing other forms of selenium, such as Na2SeO3 and Na2SeO4, in nutritional supplements [19]
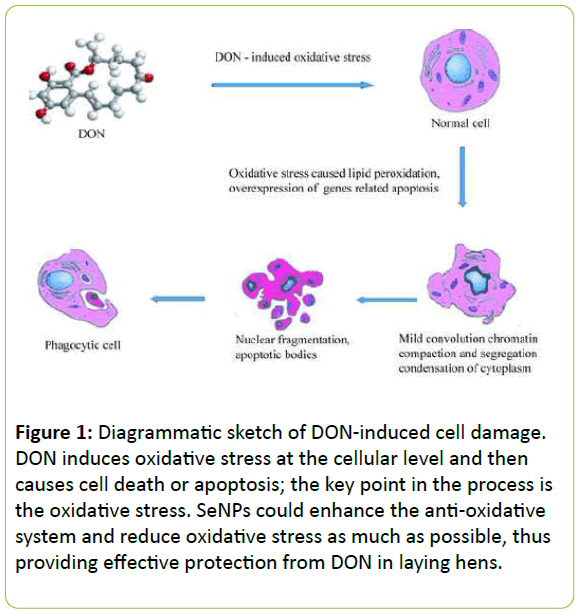
Based on the current understanding of DON toxicology and the biological function of SeNPs, we hypothesized that SeNPs may alleviate DON toxicity (Figure 1). In the present study, SeNPs were synthesized by Bacillus licheniformis, and their protective effects against DON in laying hens were examined.
Figure 1: Diagrammatic sketch of DON-induced cell damage. DON induces oxidative stress at the cellular level and then causes cell death or apoptosis; the key point in the process is the oxidative stress. SeNPs could enhance the anti-oxidative system and reduce oxidative stress as much as possible, thus providing effective protection from DON in laying hens.
Materials and Methods
Preparation of SeNPs and DON
Bacillus licheniformis, which was previously isolated from a pig farm and identified by 16S rRNA and gyrB gene analysis techniques, was used to biosynthesize SeNPs following the method described by Shakibaie et al. [22]. Briefly, 2 ml of activated Bacillus licheniformis were added to 200 mL of Luria- Bertani nutrient solution containing Na2SeO3 (final concentration of 200 μg.ml-1) and incubated in a shaker incubator (200 rpm, DAIHAN ThermoStable™, DAIHAN Scientific Co. Ltd., Korea) at 37°C for 24 h.
The color of the solution changed from clear light yellow to red. Then, the bacterial cells were harvested by centrifugation at 4,000 xg for 10 min (Kentro Laboratory Products, U.S.A.). After the obtained biomass was washed with sterile NaCl solution (0.9%) three times, the bacterial cells were then ultra-sonicated at 100 W for 30 min (Sanyo, U.K.) and washed three times by sequential centrifugation (14,000 xg, 5 min) with a 1.5 M Tris– HCl buffer (pH 8.3) containing 1% sodium dodecyl sulfate (SDS) and deionized water. The precipitate was dissolved in deionized water again and mixed with 2-octanol in 2 volumes.
Next, the mixture was centrifuged (2000 xg for 5 minutes) and stored at 4°C for 24 hours to allow the SeNPs to settle. Then, the purified SeNPs were obtained after successive washings with trichloromethane, absolute ethyl alcohol and deionized water.
Finally, the purified SeNPs were stored at 4°C, and their characteristics were examined with a transmission electron microscope (TEM, FEI Tecnai G2 spirit). DON was provided by the Institute of Agri-food Standards and Testing Technology, SAAS.
Hens and housing
Ninety-six healthy laying hens (hyline-brown, twenty weeks old) with similar initial body weights (BW) and laying rates were obtained from a commercial layer farm in Shanghai and then individually housed in a three-deck laying cage, which allowed feed ingestion and egg production to be quantified for each individual.
A basal diet was provided ad libitum for a weeklong adaptation period, during which daily individual egg production was recorded.
At the end of this pre-experimental period, the hens were individually weighed and randomly divided into four groups, each of which included 3 replicates of 8 layers, and all groups had similar average BW and egg production before the experiment. The experiment was carried out for four consecutive weeks.
The experiment involved four treatment groups: a control group (fed the basal diet), the DON group (challenged by 10 mg/kg DON in the diet), the DON+SeNPs group (challenged by 10 mg/kg DON and provided 0.5 mg/kg SeNPs) and the SeNPs group (provided 0.5 mg/kg SeNPs).
The concentrations of DON and selenium in the experiment were set based on previous studies [21-23], and the experimental diet was designed according to NRC (1994) requirements. The ingredients and chemical composition of the basal diet is shown in Table 1. All birds had free access to feed and water.
| Composition | Level (%) |
|---|---|
| Ingredients | |
| Corn | 59.35 |
| Soybean meal | 17.6 |
| Sunflower meal | 4 |
| Cottonseed meal | 3.8 |
| Corn gluten meal | 3 |
| Vegetable oil | 1.3 |
| CaHPO4 | 1.6 |
| Limestone | 8 |
| NaCl | 0.3 |
| DL-Methionine | 0.05 |
| Premix1 | 1 |
| Nutrition | |
| Dry matter | 90.32 |
| Crude protein | 16.79 |
| Crude fiber | 6.43 |
| Ether extract | 4.35 |
| Organic material | 87.64 |
| Nitrogen-free extract | 60.13 |
| Calcium | 3.76 |
| Total phosphorus | 0.68 |
Table 1: Composition and nutrient levels of the basal diet.
The animal handling protocol for this study was approved by the Animal Welfare Committee of SAAS and was conducted in accordance with the guidelines for experimental animals.
Egg production and egg quality
Throughout the experiment, the health of the hens and their egg production were monitored by feeding staff every day. The egg production rate of each group was calculated as the total number of eggs/total number of hens in each group, and the soft-shelled or cracked egg rate was calculated as the number of soft-shelled or cracked eggs/total number of eggs. On the fifth day of each week, 8 eggs were collected from each group for an egg quality test, in which egg weight, shell strength and thickness, Haugh units, yolk color and egg shape indexes were determined with automatic egg testing equipment (State Key Laboratory of Poultry, SAAS).
Assessment of the serum antioxidative level
On the last day of the experiment, 12 h after feed withdrawal, blood samples were drawn into Eppendorf tubes (10 ml) from the axillary vein of 8 hens in every group. After the serum was naturally separated, it was centrifuged 3,000 xg for 15 min, and pure serum samples were aspirated by pipette, stored in 1.5 ml Eppendorf tubes at -70°C until analysis, and thawed at 4°C before analysis. Serum total antioxidant capacity (T-AOC) and glutathione peroxidase (GPx) activity were detected using assay kits (colorimetric method) (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s protocols.
Evaluation of blood routine and biochemical indexes
Blood samples were collected on the last day of the experiment. Whole blood was used to evaluate the blood routine indexes, and pure serum was used for the biochemical indexes. Indexes were detected with a fully automatic hematology analyzer at the Institute of Animal Husbandry and Veterinary Science, SAAS.
Blood routine indexes included white blood cell count (WBC), lymphocytes (LY), monocytes (MO), granulocytes (GR), red blood cell count (RBC), hemoglobin (HGB), and mean platelet volume (MPV).
Biochemical indexes included albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), glucose (GLUC), UREA, blood calcium (Ca), total bilirubin (TBILC), creatinine (CRE) and total protein (TP).
Statistical analysis
Data were analyzed statistically by one-way ANOVA and mean comparisons in SPSS 16.0 and Graphpad Prism 16.0 for WindowsR.
Results
Biosynthesis of SeNPs with Bacillus licheniformis
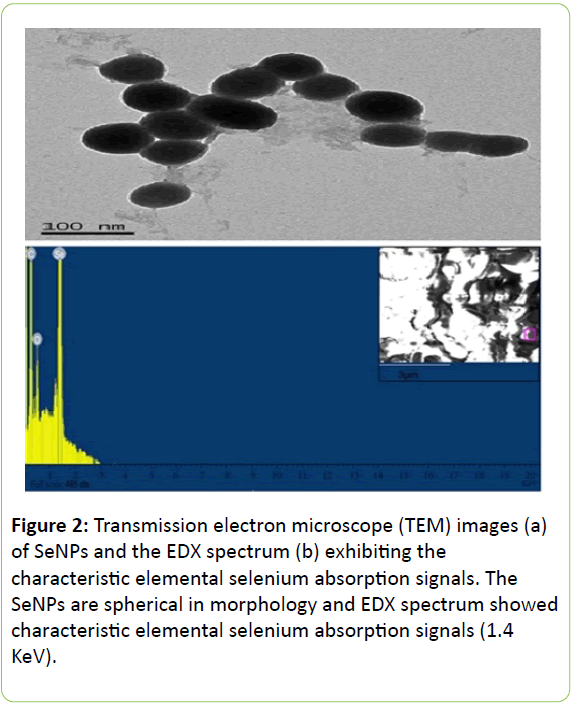
SeNPs were successfully biosynthesized by Bacillus licheniformis and purified using a 2-octanol-water partitioning system. Based on the TEM images (Figures 2a and 2b), the purified SeNPs presented spherical shapes, and most ranged from 50 to 110 nm in diameter.
In addition, energy dispersive X-ray (EDX) (OXFORD Instruments) was used to analyze the components of the biosynthesized SeNPs, and the EDX spectrum exhibited the characteristic elemental selenium (Se0) absorption signals (1.4 KeV).
Based on previous research, 5-200 nm of SeNPs showed high bioactivity and directly scavenged free radicals in vitro [23,24].
Laying performance and egg quality
No mortality was recorded during the four weeks of the experiment. Eggs in all groups had similar egg quality parameter values (egg weight, Haugh unit, yolk color, eggshell intensity, eggshell thickness and index of the egg sharp) over the entire experimental period (Tables 2 and 3).
| Items | Control | DON | DON+SeNPs | SeNPs | P-value |
|---|---|---|---|---|---|
| Egg weight (g) | 63.4±4.24 | 65.3±4.46 | 63.94±4.66 | 62.76±4.77 | 0.8087 |
| Shell strength (kgf 2) | 3.17±0.63 | 3.8±0.20 | 3.55±0.31 | 3.24±0.76 | 0.1805 |
| Yolk color (score) | 8.53±0.60 | 8.24±0.40 | 8.85±0.76 | 8.57±0.21 | 0.3078 |
| Haugh units (score) | 93.57±2.67 | 96.92±5.43 | 98.11±3.36 | 93.41±4.74 | 0.0866 |
| shell thickness 3 (mm) | 0.34±0.03 | 0.38±0.01 | 0.36±0.04 | 0.34±0.02 | 0.1309 |
| Egg shape index | 1.25±0.05 | 1.31±0.02 | 1.3±0.06 | 1.29±0.05 | 0.1215 |
2kgf=kilogram-force
3Eggshell thickness was the mean of 3 different points (blunt, middle, sharp) on the egg.
Table 2: Effect of SeNPs and DON on egg quality in the first week.
| Items | Control | DON | DON+SeNPs | SeNPs | P-value |
|---|---|---|---|---|---|
| Egg weight (g) | 61.68±6.17 | 63.4±5.43 | 65.74±4.77 | 62.05±4.24 | 0.0538 |
| Shell strength (kgf2) | 3.34±0.36 | 3.33±0.84 | 3.62±0.50 | 3.60±0.6 | 0.2743 |
| Yolk color (score) | 8.15±0.54 | 8.20±0.50 | 8.05±1.12 | 8.62±0.66 | 0.1271 |
| Haugh units (score) | 91.52±4.81 | 97.17±2.5 | 95.05±3.87 | 91.92±4.35 | 0.3009 |
| Shell thickness 3 (mm) | 0.35±0.02 | 0.36±0.02 | 0.35±0.02 | 0.34±0.03 | 0.1653 |
| Egg shape index | 1.32±0.06 | 1.31±0.03 | 1.29±0.05 | 1.3±0.04 | 0.1594 |
2kgf=kilogram-force
3Eggshell thickness was the mean of 3 different points (blunt, middle, sharp) on the egg.
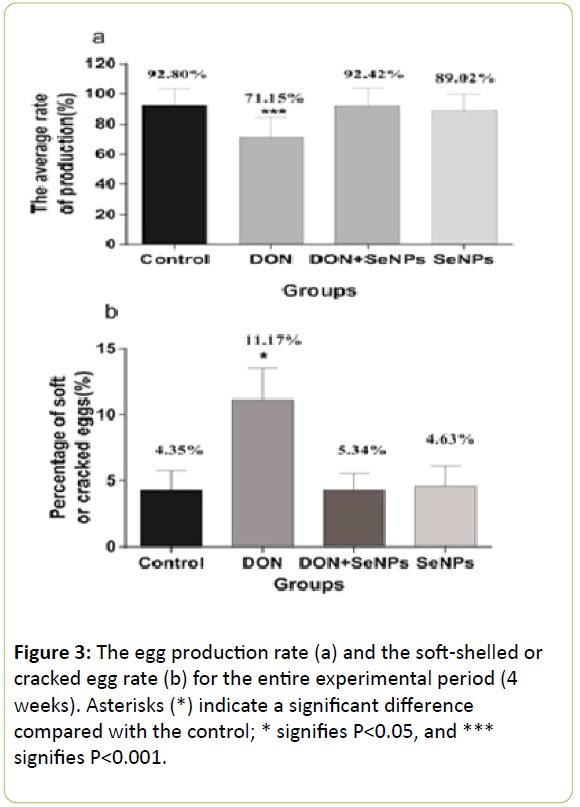
However, hens fed DON had the lowest (P<0.01) egg production and the highest (P<0.05) soft-shelled or cracked egg rates among all treatments.
In addition, there was no significant difference (P>0.05) in the laying rate and soft-shelled or cracked egg rate between the DON+SeNPs and control groups (Figure 3).
Serum antioxidative level
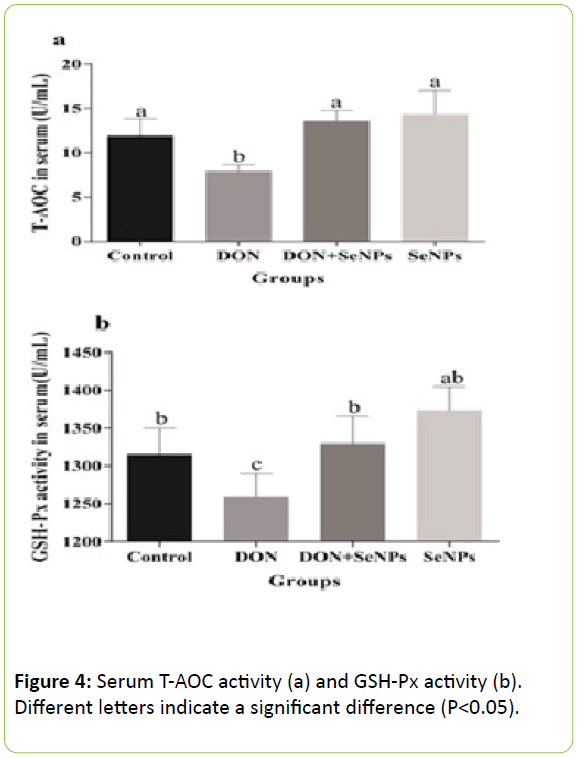
The antioxidative level in the serum is shown in Figure 4. According to the enzyme activity levels, it was concluded that the SeNPs significantly eased the oxidative stress induced by DON.
The activities of T-AOC and GPx in the serum of laying hens in the DON group were significantly (P<0.05) lower than those of laying hens in the control group. However, there was no significant (P>0.05) difference in enzyme activity between the DON+SeNPs group and the control group.
These results indicated that DON actually inhibited the activities of the enzymes related to antioxidation, and the SeNPs successfully improved the unbalanced oxidative state caused by DON.
Blood routine and biochemical indexes
Of the blood routine indexes (Table 4), WBC, LY, MO and GR in the DON group were significantly (P<0.05) decreased, and RBC, HGB and MPV were significantly (P<0.05) increased compared with control group. These indexes in the DON+SeNPs and the SeNPs groups did not differ significantly from the control. Of the biochemical indexes, serum calcium significantly (P<0.05) decreased in the DON group, but there were no significant changes in the other indexes compared with the control group (Table 5).
| Parameter | Control | DON | DON+SeNPs | SeNPs |
|---|---|---|---|---|
| WBC (´10 9/L) | 9.56±1.08 a | 6.30±2.04 b | 8.61±2.30 a | 7.90±0.63 a |
| LY (´10 9/L) | 1.09±0.13 a | 0.75±0.21 b | 1.00±0.25 a | 0.93±0.09 a |
| MO (´10 9/L) | 0.49±0.06 a | 0.32±0.10 b | 0.43±0.11 a | 0.41±0.03 a |
| GR (´10 9/L) | 8.02±0.90 a | 5.28±1.75 b | 7.24±1.93 a | 6.61±0.50 a |
| RBC (´10 12/L) | 2.37±0.09 b | 3.26±1.18 a | 2.49±0.44 b | 2.50±0.14 b |
| HGB (g/L) | 170.50±8.99 b | 234.63±80.97 a | 178.43±35.37 b | 185.25±10.32 b |
| MPV (fL) | 4.98±0.29 b | 7.17±1.03 a | 5.73±0.36 b | 5.99±0.23 b |
Table 4: Effects of SeNPs and DON on blood routine indexes.
| Parameter | Control | DON | DON+SeNPs | SeNPs |
|---|---|---|---|---|
| ALB (g/L) | 16.58±0.89 | 17.05±1.83 | 16.67±2.17 | 16±1.49 |
| AST (U/L) | 190.74±15.4 | 204.3±34.18 | 186.71±17.07 | 188.39±20.07 |
| ALT(U/L) | 2.89±1.37 | 2.75±1.91 | 3.49±1.54 | 3.36±1.76 |
| GLUC (mmol/L) | 0.49±0.14 | 0.41±0.1 | 0.39±0.04 | 0.45±0.15 |
| UREA (mmol/L) | 0.49±0.14 | 0.41±0.1 | 0.39±0.04 | 0.45±0.15 |
| Ca (mmol/L) | 7.92 ±0.69 a | 6.38±1.03 b,c | 7.17±1.14 a,b | 7.26±0.85 a,b |
| TBILC (μmol/L) | 2.25±0.46 | 1.91±0.49 | 1.91±0.4 | 1.94±0.4 |
| CRE (μmol/L) | 6±2.67 | 5±1.77 | 5.29±0.95 | 5±1.07 |
| TP (g/L) | 54.45±3.16 | 57.45±14.45 | 51.99±5.08 | 52.48±4.2 |
Table 5: Effects of SeNPs and DON on blood biochemistry indexes.
Discussion
Over the last decade, DON-induced oxidative stress has been a hot topic of research, and many studies have revealed the role of oxidative stress in DON toxicity in animals and humans [6]. DON could induce oxidative stress in several cell lines, such as chicken embryo fibroblast DF-cells [30], human peripheral blood lymphocytes [25] and murine YAC-1 lymphoma cells [18], thus causing cell lipid peroxidation, DNA fragmentation, or apoptosis.
GPx and T-AOC are the main antioxidizing enzymes that protect biological membranes and the large biological molecules from oxidative tissue damage. In this research, the levels of these enzymes were significantly (P<0.05) inhibited by DON compared with the control, indicating that DON could induce oxidative stress in laying hens. This result is consistent with the previous research described above, and studies have also found that oxidative stress affects the female reproductive system by inhibiting follicle cell growth [26,27] or by even damaging ovarian and uterine cells. This is the probable explanation for why the DON group exhibited a lower egg production rate (71.15%) compared with the other groups.
According to blood routine indexes, we found that WBC, LY, MO and GR significantly (P<0.05) decreased in the DON group, meaning that DON inhibited the immune system of the hens. DON also significantly (P<0.05) decreased serum calcium, which is essential for strong bones and teeth. In laying hens, calcium is required for egg production, so the decrease in serum calcium significantly (P<0.05) increased the soft-shelled or cracked egg rate (11.17%) compared with the other groups.
Selenium, as a necessary and biogenic trace element, can enhance antioxidant activity, and this has been supported by both clinical and laboratory experiments. The selenium nanoparticle (SeNP), which is one form of elemental selenium (Se0), has lower toxicity and better absorption and it is most effective when provided to broiler chicken under oxidative stress with the same dose but different sources of Se (organic, inorganic and nano-Se) [28]. According to some studies, the size of SeNPs synthesized by microorganisms has mostly been between 20 and 300 nm [20,26,27], and the diameter of the SeNPs in this research was 50 to 110 nm, which is consistent with previous research.
In this study, SeNPs significantly increased GPx and T-AOC activity, improved egg production (92.42%) and decreased the soft-shelled or cracked egg rate (5.43%) compared with the DON group (11.17%). This likely occurred for two reasons: the SeNPs reduced the oxidative stress in the bodies of the hens, and selenium improved the status of the avian immune system by increasing the ability of immunocompetent cells to respond to an antigen challenge (such as DON in the present study). Scheideler et al. found that selenium could improve the vitelline membrane strength of fresh and aged eggs while also increasing the levels of these nutrients in the egg yolk [29]. The protection of SeNPs against DON in this study is also consistent with Rizzo, who found that a selenium pretreatment provided protection against acute DON toxicity in male rats [30].
Conclusion
The results of this study showed that SeNPs have the potential to alleviate DON toxicity in laying hens by reducing DON-induced oxidative stress, and they might be used as a novel anti-mycotoxin agent.
Acknowledgements
The research was completed with the financial aid of the Developing Agriculture through Science and Technology Key Project of the Shanghai Agricultural Commission (Project Number: Agricultural word 2013-3-8) and the National Science and Technology Support Plan Projects of China (2015BAD11B02). We are grateful to the Institute of Animal Husbandry and Veterinary Science and the Institute of Agri-food Standards and Testing Technology, Shanghai Academy of Agricultural Sciences (SAAS) for providing the necessary equipment. The authors also thank all personnel who assisted with the studies.
References
- Pestka JJ (2007) Deoxynivalenol: Toxicity, mechanisms and animal health risks. Animal Feed Science and Technology 137: 283-298.
- Zhang X, Jiang L, Geng C, Cao J, Zhong L (2009) The role of oxidative stress in deoxynivalenol-induced DNA damage in HepG2 cells. Toxicon 54: 513-518.
- Pestka JJ, Smolinski AT (2005) Deoxynivalenol: toxicology and potential effects on humans.J Toxicol Environ Health B Crit Rev 8: 39-69.
- Prelusky DB, Gerdes RG, Underhill KL, Rotter BA, Jui PY, et al. (1994) Effects of low-level dietary deoxynivalenol on haematological and clinical parameters of the pig. Nat Toxins 2: 97-104.
- Awad WA, Aschenbach JR, Setyabudi FM, Razzazi-Fazeli E, Böhm J, et al. (2007) In vitro effects of deoxynivalenol on small intestinal D-glucose uptake and absorption of deoxynivalenol across the isolated jejunal epithelium of laying hens. PoultSci 86: 15-20.
- Mishra S, Dwivedi PD, Pandey HP, Das M (2014) Role of oxidative stress in Deoxynivalenol induced toxicity. See comment in PubMed Commons below Food ChemToxicol 72: 20-29.
- Braicu C, Berindan-NeagoeI, Tudoran O.B.O, Rugina D, Gherman C, et al. (2009) In vitro evaluation of the chemoprotective action of flavan-3-ols against deoxynivalenol related toxicity. Arch. Zootechn 12:3 45-55.
- Costa S, Schwaiger S, Cervellati R, Stuppner H, Speroni E, et al. (2009) In vitro evaluation of the chemoprotective action mechanisms of leontopodic acid against aflatoxin B1 and deoxynivalenol-induced cell damage. J ApplToxicol 29: 7-14.
- Sahu SC, O'Donnell MW, Wiesenfeld PL (2010) Comparative hepatotoxicity of deoxynivalenol in rat, mouse and human liver cells in culture. ApplToxicol 30: 566-573.
- Li D, Ye Y, Lin S, Deng L, Fan X, et al. (2014) Evaluation of deoxynivalenol-induced toxic effects on DF-1 cells in vitro: cell-cycle arrest, oxidative stress, and apoptosis. Environ ToxicolPharmacol 37: 141-149.
- Sahu SC, Garthoff LH, Robl MG, Chirtel SJ, Ruggles DI, et al. (2008) Rat liver clone-9 cells in culture as a model for screening hepatotoxic potential of food-related products: hepatotoxicity of deoxynivalenol. J ApplToxicol 28: 765-772.
- Kouadio JH, Dano SD, Moukha S, Mobio TA, Creppy EE (2007) Effects of combinations of Fusariummycotoxins on the inhibition of macromolecular synthesis, malondialdehyde levels, DNA methylation and fragmentation, and viability in Caco-2 cells. Toxicon 49: 306-317.
- Frankic T, Salobir J, Rezar V (2008) The effect of vitamin E supplementation on reduction of lymphocyte DNA damage induced by T-2 toxin and deoxynivalenol in weaned pigs. Animal Feed Science and Technology 141: 274-286.
- Frankic T, Pajk T, Rezar V, Levart A, Salobir J (2006) The role of dietary nucleotides in reduction of DNA damage induced by T-2 toxin and deoxynivalenol in chicken leukocytes. Food ChemToxicol 44: 1838-1844.
- Awad WA, Ghareeb K, Dadak A, Gille L, Staniek K, et al. (2012) Genotoxic effects of deoxynivalenol in broiler chickens fed low-protein feeds. PoultSci 91: 550-555.
- Kalaiselvi P, Rajashree K, BharathiPriya L, Padma VV (2013) Cytoprotective effect of epigallocatechin-3-gallate against deoxynivalenol-induced toxicity through anti-oxidative and anti-inflammatory mechanisms in HT-29 cells. Food ChemToxicol 56: 110-118.
- Krishnaswamy R, Devaraj SN, Padma VV (2010) Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: prevention of NF-kappaB nuclear localization and down regulation of NF-kappaB and Cyclo-Oxygenase-2 expression. Free RadicBiol Med 49: 50-60.
- Strasser A, Carra M, Ghareeb K, Awad W, Böhm J (2013) Protective effects of antioxidants on deoxynivalenol-induced damage in murine lymphoma cells. Mycotoxin Res 29: 203-208.
- Jia X, Li N, Chen J (2005) A subchronic toxicity study of elemental Nano-Se in Sprague-Dawley rats. Life Sci 76: 1989-2003.
- Wang Y, Wang J, Hao H, Cai M, Wang S, et al. (2016) In Vitro and in Vivo Mechanism of Bone Tumor Inhibition by Selenium-Doped Bone Mineral Nanoparticles. ACS Nano 10: 9927-9937.
- Wang Y, Hao H, Liu H, et al. (2015) Selenite-Releasing Bone Mineral Nanoparticles Retard Bone Tumor Growth and Improve Healthy Tissue Functions In Vivo. AdvHealthc Mater 4: 1813-1818.
- Shakibaie M, Forootanfar H, Golkari Y, Mohammadi-Khorsand T, Shakibaie MR (2015) Anti-biofilm activity of biogenic selenium nanoparticles and selenium dioxide against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis. J Trace Elem Med Biol 29: 235-241.
- Peng D, Zhang J, Liu Q, Taylor EW (2007) Size effect of elemental selenium nanoparticles (Nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activity. J InorgBiochem 101: 1457-1463.
- Forootanfar H, Adeli-Sardou M, Nikkhoo M, Mehrabani M, Amir-Heidari B, et al. (2014) Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. J Trace Elem Med Biol 28: 75-79.
- Yang W, Yu M, Fu J, Bao W, Wang D, et al. (2014) Deoxynivalenol induced oxidative stress and genotoxicity in human peripheral blood lymphocytes. Food ChemToxicol 64: 383-396.
- Margolin Y, Aten RF, Behrman HR (1990) Antigonadotropic and antisteroidogenic actions of peroxide in rat granulosa cells. Endocrinology 127: 245-250.
- Tsai-Turton M, Luderer U (2006) Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology 147: 1224-1236.
- Boostani A, Sadeghi AA, Mousavi SN, Chamani M, Kashan N (2015) Effects of organic,inorganic,and nano-Se on growth performance,antioxidant capacity,cellular and humoral immune responses in broiler chickens exposed to oxidative stress. Livestock Science 178: 330-336.
- Scheideler SE, Weber P, Monsalve D (2010) Supplemental vitamin E and selenium effects on egg production, egg quality, and egg deposition of a tocopherol and selenium. J ApplPoult Res 19: 354-360.
- Rizzo AF, Atroshi F, Ahotupa M, Sankari S, Elovaara E (1994) Protective effect of antioxidants against free radical-mediated lipid peroxidation induced by DON or T-2 toxin. ZentralblVeterinarmed A 41: 81-90.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences