Effects of Foliar Fungicide Application on Corn Plants on the Composition of Corn Silage for Ruminant Diets
Kalebich CC, Cardoso FC
DOI10.21767/2572-5459.100025
Kalebich CC and Cardoso FC*
Department of Animal Sciences, University of Illinois, Urbana, IL, USA
- *Corresponding Author:
- Cardoso FC
Department of Animal Sciences, University of Illinois
1207 W Gregory Drive, Urbana, IL-6180, USA
Tel: 2173002303
Fax: 217.333.7088
E-mail: cardoso2@illinois.edu
Received date: October 10, 2016; Accepted date: May 10, 2017; Published date: May 15, 2017
Citation: Cardoso FC (2017) Effects of Foliar Fungicide Application on Corn Plants on the Composition of Corn Silage for Ruminant Diets. J Anim Res Nutr 2017, 2:5. doi: 10.21767/2572-5459.100025
Copyright: © 2017 Cardoso FC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
An increasing global population, decreasing amount of arable land available for crop production in the United States, and an increased global demand for protein in the human diet encourage crop and livestock producers to seek solutions to improve the efficiency of producing large crop yields. Corn silage is one of the most commonly used forages included in dairy diets in the United States. For producers, feed costs are often the most expensive part of the operating budget. Corn yield losses may increase the cost of feed and limit herd size. The complex interaction of fungi and corn plants in the field threaten yields, decreasing the efficiency of food production and, also, the nutritive quality and value of feedstuffs for ruminants. By metabolizing sugar compounds within the plant cell, fungal infections on corn plants reduce the nutritional contents available for ruminant diets. Applications of fungicides can aid in protecting corn plants from fungal infection, therefore, limiting yield losses and increasing the nutritive quality of the plant material. The field of knowledge of feeding ruminants corn silage from corn treated with foliar fungicide is still narrow, but findings from previous research highlight the negatives of making and feeding silage from diseased corn plants. This review will summarize the knowledge available on fungi and plant relationship, limiting plant infection by applying fungicide, and how corn silage from corn with fungicide application affects dairy cow performance. It is concluded fungicide application on corn used to make corn silage for dairy cows may improve the nutritional composition of the feedstuff, as defined by increases in milk components and feed conversion, reductions in fiber concentrations, and improvements in ruminal digestibility.
Keywords
Corn; Fungicide; Corn silage; Fungus
Introduction
Increases in the global efficiency of converting cropland to livestock products (milk, meat, and eggs) could help lessen the caloric dependency on cereal grain, which is required to nourish the world’s growing population [1]. On a DM basis, whole plant corn silage composition is about 57% corn ears, 13% corn leaves, and 31% corn stalks [2]. For dairy cow diets, corn silage represents 40–60% of the total mix ration in lactating diets [3]. But, fungi can also have a parasitic relationship with corn plants. Under certain weather conditions, fungal pathogens on the growing plant complete the last side of the disease triangle between host, pathogen, and environment. Both physical barriers such as cell walls, and chemical releases such as secondary metabolites aid plants in protecting from pathogens [4]. If fungi remain undetected on the plant surface, enzymes degrade cell walls and once inside produce toxins killing the plant tissue, thus providing nutrients for fungal growth [5]. Plants have adapted by increasing the lignin concentration in the secondary cell wall, thus creating a tougher barrier for digesting when wounded or infected with a fungal pathogen or insect [6]. However, once inside the cell and growth has ceased, fungal pathogens release secondary metabolites, which in some species are toxic. It is generally hypothesized during the colonization and sporulation phase of a fungus within a plant; mycotoxins are secreted by growing colonies [7]. Few studies have been conducted on how foliar fungicide applications on diseased corn affect the nutritive quality of various parts of the plant.
Fungus-A Threat for Corn
Yield losses due to fungal infections
In 2013, 7.5% of the total estimated corn harvested from 21 corn producing states was lost to disease; meaning nearly 27 million metric tons of corn was lost because of seedling blights and foliar diseases [3]. Under ideal weather conditions for pathogenesis, a 1% increase in foliar disease severity of Gray leaf spot, caused by the fungus Cercospora zeaemaydis, reduced corn yields by 47.6 kg/ha when compared with a tolerant hybrid [8,9]. Furthermore, in a meta-analysis of 20 studies, every 10% increase in rust severity on sweet corn, caused by the fungus Puccinia sorghi, reduced corn yields 2.4 to 7.0% [10]. Mycotoxins, a secondary metabolite of fungi, contaminated 12.5% of the total harvested grain in the United States in 2013; mostly because of the disease Aspergillus ear rot caused by the fungi Aspergillus flavus and A. parasiticus [11]. Evidence shows fungal infection and disease on plants can cause devastating losses in corn yield [3].
Fungal relationship with plants
All fungal-plant relationships are not parasitic. Most fungi associated with plants are saprotrophs, responsible for decomposing organic matter as their food source [12]. Other fungi, about 160 known species, reside on the roots of growing plants in a mutualistic relationship. Carbohydrates produced by the plant feed the fungus, and the fungus transports nitrogen, phosphorous, and other minerals to the plant [12]. A very small amount of fungi are disease causing, totaling less than 10% of about 100,000 known species, that colonize plants [13]. For instance, Meng et al. has reported that inoculating with both Arbuscular mycorrhizal fungi and Rhizobium in the soybean/ maize intercropping system improved the N fixation efficiency of soybean and promoted N transfer from soybean to maize, resulting in the improvement of yield advantages of legume/ non-legume intercropping [14].
Disease triangle
Plant pathologists use the disease triangle for assistance when evaluating the likelihood of a disease outbreak. A susceptible host (plant), a pathogen, and a favorable environment are all necessary for development of plant infection, presence of just two is unlikely to result in disease. The relationship between fungi and plants is sometimes referred to as an “arm’s race” [4].
Pathogen
By definition, pathogens cause disease and need to complete their life cycle within the host [5]. Historically, fungi can be divided into two main groups, both of which originate in the field. Field fungi produce toxins in the plant before harvest and are governed by a plant-fungus interaction. Fungus can be a problem postharvest, and a function of crop nutrients, physical, and biotic factors [11].
Once in the cell, the fungal pathogen either adapt to the host’s physiology or modify the environment for nutrient uptake to allow for colonization within the host [13,5]. Once fungal pathogens invade, plant cell oxidative bursts signal other metabolic pathways of an invasion but in doing so locally kill plant tissue providing immediate nutrients to the fungus [4,5]. For more long-term nutrition, a haustoria, a specialized fungal structure, can be inserted into the plant cell for water and nutrient uptake, especially hexose carbohydrates including sucrose, glucose and fructose [15]. The diversion of plant nutrients can be used for fungal growth and development.
Once inside the cell and growth has ceased, fungal pathogens release secondary metabolites, which in some species are toxic. It is generally hypothesized during the colonization and sporulation phase of a fungus within a plant, mycotoxins are secreted by growing colonies [7]. The exact function of fungal toxins in the plant is unclear. Fungal phytotoxins can cause direct plant cell death by over activation of the plant plasma membrane enzyme, H+ATPase, which disrupts energy transfer during the light reactions in the chloroplasts the closing or opening of the stomata, and the redirection of ion channels [5,13,16]. But agriculturally, mycotoxins threaten food safety and security.
Five agriculturally important mycotoxins resulting from corn ear rot include: deoxynivalenol, from the fungus Fusarium graminearum; zeralenone from the fungus F. graminearum; ochratoxin A from the fungi Piper verrucosum and A. ochraceus; fumonisin from the fungus F. moniliforme; and aflatoxin from the fungi A. flavus and A. parasiticus [11]. Development of mycotoxins within the plant occurs later in the growth and development of the corn plant. One study showed fumonisin concentration within corn kernels increased greatly as the corn plant became more mature, with only 33% of corn kernels infected at the fourth reproductive stage, but 62.5% of corn kernels infected at harvest [17]. Furthermore, while it is generally thought tilling fields may reduce fungi colonization it may not be the case as Ariño et al. showed no difference in fumonisin concentrations in varying degrees of tilled fields [17].
Host
The cell wall is the next physical barrier to fungal invasion. The plant cell wall is composed of a primary cell wall, providing structural support for the plant, and a secondary cell wall, developing inside the primary cell wall only after the plant cells stop growing [18]. The primary wall of plant cells is composed of cellulose, cross-linking glycans, also known as hemicellulose, and pectins. Cellulose is a polysaccharide, comprised of β (1,4)- glyosidic bonds between glucose molecules and very resistant to degradation by hydrolysis. Hemicellulose is also a polysaccharide, where a pentose is bonded with a hexose, e.g. arabinoxylans, xyloglucans, mixed linked beta glucans, and galactomannans. The cross-linking of hemicellulose aids in the fortification of cellulose for both structural support and prevention of microbial invasion. Enzymes such as xylanase, produced by some fungi, weaken the cell wall and allow fungal entry into the plant cell. Lignin, a phenolic polymer, is deposited during the last stages of secondary cell wall formation. Lignin reinforces plant cells and allows transport of water under negative cellular pressure [4]. When cell walls become lignified, it becomes highly impermeable to pathogens and hard for insects to digest, limiting access to cell wall sugars [4,18].
Plants have a recognition system controlled by resistance genes within the plant cell known as plant triggered immunity (PTI) [5]. ‘Pathogen associated molecular patterns’, also known as PAMPs which may include fungal chitin or bacteria flagellin, can trigger a PTI response within the plant cell to prevent microbial colonization [4]. Also, ‘damage associated molecular patterns’ known as DAMPs, which may include parts of the plant cell wall released possibly due to fungal enzymes, trigger an immune reaction [4]. An activated PTI in a plant cell may cause localized death, an oxidative burst of reactive oxygen species to signal neighboring cells of invasion a rapid fluctuation in the calcium gradient to signal that a pathogen has been detected release of pathogenesis related enzymes including chitinase, to degrade fungal chitin, activation of enzymes to strength the cell wall, activation of defense genes, and induction of phytoalexins, which are antimicrobial substance synthesized de novo [4,5,18,13].
Environment
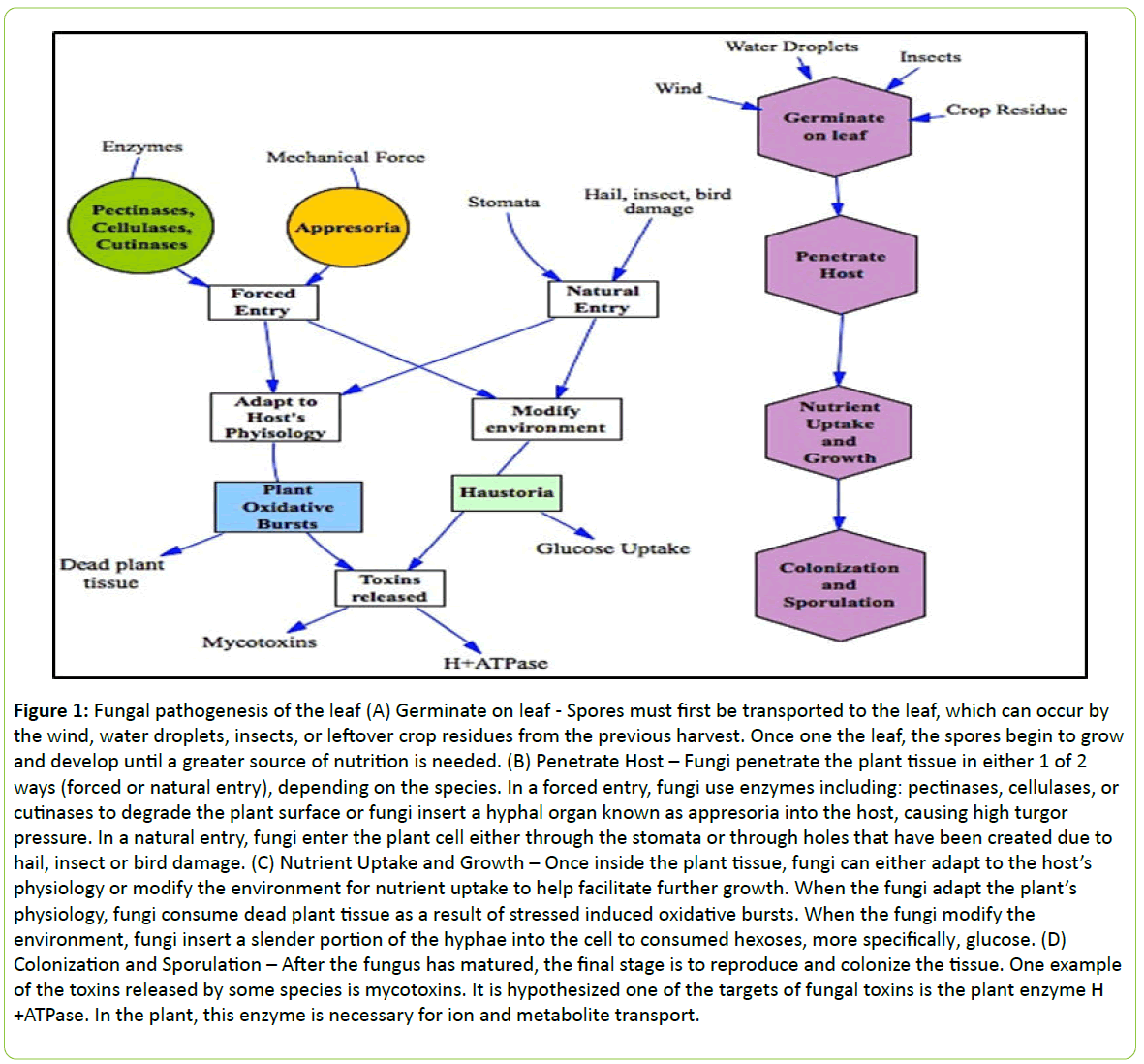
A favorable environment is needed for the development of plant disease, completing the final side of the disease triangle. The favorable environment for one species of fungi may be different for another species of fungi. For example, when growing conditions for corn include a warm ambient temperature and drought conditions, corn is more susceptible to the fungus A. flavus and A. parasiticus, which produce alflatoxin as a secondary metabolite [19]. Yet, the foliar fungus Exserohilum turcicum, causing Northern Leaf Blight in corn, favors cool and humid conditions for colonization of foliage [20]. Understanding the role the complex relationship between plant cells, fungi, and the environment is crucial for the future production of corn and those whom consume it. Figure 1 summarizes the aforementioned interactions.
Figure 1: Fungal pathogenesis of the leaf (A) Germinate on leaf - Spores must first be transported to the leaf, which can occur by the wind, water droplets, insects, or leftover crop residues from the previous harvest. Once one the leaf, the spores begin to grow and develop until a greater source of nutrition is needed. (B) Penetrate Host – Fungi penetrate the plant tissue in either 1 of 2 ways (forced or natural entry), depending on the species. In a forced entry, fungi use enzymes including: pectinases, cellulases, or cutinases to degrade the plant surface or fungi insert a hyphal organ known as appresoria into the host, causing high turgor pressure. In a natural entry, fungi enter the plant cell either through the stomata or through holes that have been created due to hail, insect or bird damage. (C) Nutrient Uptake and Growth – Once inside the plant tissue, fungi can either adapt to the host’s physiology or modify the environment for nutrient uptake to help facilitate further growth. When the fungi adapt the plant’s physiology, fungi consume dead plant tissue as a result of stressed induced oxidative bursts. When the fungi modify the environment, fungi insert a slender portion of the hyphae into the cell to consumed hexoses, more specifically, glucose. (D) Colonization and Sporulation – After the fungus has matured, the final stage is to reproduce and colonize the tissue. One example of the toxins released by some species is mycotoxins. It is hypothesized one of the targets of fungal toxins is the plant enzyme H +ATPase. In the plant, this enzyme is necessary for ion and metabolite transport.
Fungicides
Countries around the world seek to control fungal pathogens through various methods, including fungicide application on plants, in hopes that chemical application will alleviate their impact on corn. In keeping with the disease triangle, fungicide’s aid in the plants defense from fungal invasion. The Food and Agricultural Organization estimated in 2013 that Brazil applied the most fungicide on crops, using 40 thousand tons of active ingredients, followed by Mexico and then Spain, using 38 thousand and 29 thousand tons of active ingredients, respectively. In 2007, producers in the United States applied 20 thousand tons of active ingredients on crops [21].
Classes of fungicides and mode of action
Strobilurins fungicides, also known as QoI fungicides, are natural chemical structures isolated from the genera Strobilurus, in wood-rotting mushrooms. Since natural strobilurins break down quickly in UV light, synthetic analogs were developed for disease control [22]. Strobilurin fungicides are broad-spectrum fungicides, meaning the fungicide controls a wide array of fungal diseases in a variety of crops including cereals, fruits, vegetables, tree nuts, turf grasses, and ornamentals. Strobilurins bind to the quinol oxidation (Qo) site of cytochrome b. This binding stops the electron transport between cytochrome b and cytochrome c, stopping the oxidation of nicotinamide adenine dinucleotide (NADH) and synthesis of adenosine triphosphate (ATP). Once on the waxy leaf surface, strobilurins move throughout the plant either translaminarly and/or systemically [23].
A second group of fungicide commonly used today is carboxamide fungicides, also referred to as succinate dehydrogenase inhibitors (SDHI). Within the SDHI class of fungicides is the active ingredient fluxapyroxad. Succinate dehydrogenase inhibitors are broad-spectrum fungicides and can have translaminar or systemic activity within the host, depending on the pathogen and host [24].
A third group of fungicide is known as the demethylation inhibitors (DMIh) or sterol biosynthesis inhibitors (SBIs), which contain the triazole fungicides. Within the triazole class, is the active ingredient metconazole. Demethylation inhibitor fungicides are systemic and single-site specific inhibitors commonly used on cereal grain [25,26].
Benefits of fungicide application
In recent years, some researchers and chemical companies have concluded foliar fungicide application on corn may increase yields even in the absence of disease [27]. In the U.S. Corn Belt, several foliar diseases are of concern, depending on the production region, but gray leaf spot (GLS), caused by Cercospora zeaemaydis, have been the disease of greatest concern since first becoming a problem in the 1980s and 1990s. The elevation of GLS from a disease of secondary importance to a major problem throughout the eastern United States and the Midwest paralleled the adoption of reduced tillage [28].
In a meta-analysis on yield response and pyraclostrobin fungicide treatment, the mean difference in yield for plots treated with foliar fungicide increased 255.91 kg/ha compared with untreated plots [29].
Yet, even so some researchers are not entirely convinced applications increase yields the same in every field. Paul et al. concluded that when disease (e.g.; GLS) in the field is <5% the likelihood of an advantageous yield bump and beneficial physiological response enough to cover the cost of applying the fungicide is not as likely. But when disease in the field is >5%, fungicide application is more helpful by limiting yield losses due to fungal infection. Furthermore, in a consecutive two-year study Bradley and Ames did not see an increase in yield in 2008, under low disease severity environments, but in 2007 did see a yield increase when under higher disease severity. Routine scouting for disease in the cornfield is crucial for determining when fungicide application will be most profitable [30].
When a producer’s field is diseased, proper timing of fungicide application on the plant may also provide beneficial results. Under pressure from fungal disease, application of pyraclostrobin (Headline, BASF Corp.) on corn at VT (vegetative stage tassel) increased yield by 550 kg/ha compared to untreated fields of corn [31]. But others (Mueller and Pope; Wright et al., have shown earlier applications to be beneficial as well. In a year with high incidence of common rust, foliar fungicide applied as a preventative at vegetative stage six (V6), when six leaf collars are visible on the growing plant (Mueller and Pope, 2009), increased corn grain yield by 362.9 kg/ha compared to application at pre-tassel, when 6% of the total leaf area was diseased [32,33]. Yet, in a different year of the same study, when disease incidence was low, foliar fungicide applied as a preventative at V6 did not increase corn grain yield when compared to application at tassel (Wright et al.) [33].
Lastly, fungicide applications on corn may increase the concentration of nutrients within the plant material. In 2007, the University of Wisconsin reported a possible trend (0.20>P>0.10) for 1 percentage unit decrease (40.6 vs. 39.6%) in neutral detergent fiber (NDF) concentration when comparing corn silage from corn treated with foliar fungicide application, compared to untreated corn [34]. Yates et al. proposed that when a corn plant had a fungal infestation of the root, the structural components and rigidity increased, which the authors attributed to the plant attempting to decrease further infestation into the upper portion of the plant by increasing the structurally rigid components of the plant such as lignin [35].
Furthermore, a study at the University of Illinois evaluated the effects of fungicide application on the physical and nutritional content of corn plant leaves, ears, stalks, and flag leaves [36]. Fungicide applications on corn during the summer of 2015 were as follows: control (CON), corn receiving no foliar fungicide application; treatment 1 (V5), where corn received a mixture of pyraclotrobin and fluxapyroxad (PYR+FLUX) foliar fungicide at V5; treatment 2 (V5+R1), where corn received two applications of foliar fungicide, a mixture of PYR+FLUX at V5 and a mixture of pyraclostrobin+metconazole (PYR+MET) foliar fungicide at corn reproductive stage 1 (R1); treatment 3 (R1), in which corn received one application of PYR+MET foliar fungicide at R1. Corn plants with fungicide treatment were taller compared with untreated (2.7, 2.9, 3.0, and 2.9 m for CON, V5, V5+R1, and R1). Corn leaves in V5+R1 and in R1 had less yellow lower leaves than in CON and V5 (0.85, 0.77, 0.42, and 0.44 leaves for CON, V5, V5+R1, and R1, respectively). Corn stalks in V5+R1 had greater lignin concentration compared with CON and R1 (46, 56, 64, and 50 g/kg DM for CON, V5, V5+R1, and R1). Corn leaves in V5+R1 had lower acid detergent fiber (ADF) and NDF concentrations (283 and 524 g/kg for ADF and NDF, respectively) compared with leaves in CON 333 and 569 g/kg for ADF and NDF, respectively [36].
Mycotoxin and fungicide
Fungicides have been tested for preventing fungal colonization and mycotoxin contamination in cereal grains. Results of studies have been conflicting in their ability to control mycotoxin concentration within crops. Authors reported mycotoxin presence in corn silage samples without visual observation of fungus noted in the corn in the field [37]. Interestingly, a study done by Eckard et al. concluded that when corn was diagnosed visually only 1 to 3% of corn showed signs of infection on the surface; however, when the corn particles were plated it was found that the average Fusarium incidence was 46%. Corn harvested for our study could have been infected, even though visual symptoms were not present. Applications of metconazole and tebuconazole, another active ingredient of fungicide, reduced concentrations of Deoxynivalenol (DON; a Fusarium mycotoxin) and head blight in winter wheat more than applications of azoxystrobin, another active ingredient [38]. But some researchers hypothesize fungicides act as an additional stress factor for the fungus and stimulate mycotoxins as a defense mechanism [39].
Some toxigenic fungal species which may affect plants before and after harvest are P. nordicum and P. verrucosum [40]. These Penicillia are able to produce the mycotoxins ochratoxin and citrinin [40]. Both toxins have polyketide backbones and are structurally highly related. Ochratoxin A as well as citrinin are mainly nephrotoxic and hepatotoxic and may act synergistically [41]. For ochratoxin A, which is rated as a class B carcinogen, regulatory limits have been set in several countries. The level of citrinin is not currently regulated. P. verrucosum adapts its secondary metabolite profile depending on the environmental conditions [42]. For example oxidative stress usually induces defensive reactions such as radical scavenging mechanisms for reactive oxygen species (ROS). Schmidt-Heydt et al. reported that the biosynthesis of the mycotoxins, ochratoxin A/B and citrinin were strongly induced when grown on malt extract glucose agar medium supplemented with the fungicide Rovral (Bayer Crop Science, Germany) [40].
Fungal contamination of corn silage can lead to DM loss, nutrient loss, and reduced palatability [43]. Foliar pathogens decrease the area of photosynthetic tissue, which reduces the transfer of assimilates to grain production by diverting assimilates to fungal growth, defense systems, and increased respiration [44]. Whitlock et al. reported that feeding spoiled corn silage from the surface of a bunker silo depressed nutrient digestibility and DM intake of steers [45]. Gerlach et al. fed spoiling silage to goats and reported negative correlations between ethyl-lactate and ethanol with dry matter intake but the strongest negative relationship with intake was from silage temperature [46]. A variety of mycotoxins produced from fungi can be found in silages that are fed to dairy cattle [47]. Their presence is undesirable because they have the potential to induce negative effects on the health of animals [48]. Mycotoxins can accumulate on the plant in the field before harvest (Doerr) during storage, or during processing or feeding [49,50]. Korosteleva et al. reported that Fusarium mycotoxins decreased some cellular aspects of immune function in dairy cattle, while stimulating primary humoral response to specific antigens. The authors concluded that feeding of contaminated materials to dairy cows should be minimized [51]. Nonetheless, Charmley et al. reported that the inclusion of the mycotoxin DON in the diet of primiparous dairy cows yielding 20 to 25 kg/d milk, at up to 6 mg/kg total diet DM over a 10-week period, had no effect on volume of milk produced [52].
Cost-benefit relationships
Application of fungicide to assist in fungal control on corn costs producers money. Paul et al. calculated a 10-year average corn grain price of $0.12/kg ($2.97/bushel) and application costs of $40 to 95/ha, and showed that the probability of failing to recover the fungicide application cost (Ploss) for disease severity <5% was 0.55 to 0.98 for pyraclostrobin. However, when disease severity was >5%, the corresponding probability was 0.36 to 95. They concluded that the high Ploss values found in most scenarios suggest that the use of these foliar fungicides is unlikely to be profitable when foliar disease severity is low and yield expectation is high [29].
However, some of value may be returned to producers by increasing the efficiency of converting feed to milk when feeding feedstuffs with fungicide application in the field to dairy. During the 2014 growing season, corn was sprayed with foliar fungicide either once, twice, three times or not at all and ensiled as corn silage. Then during the summer months of 2015, dairy cattle were fed corn silage from corn treated with foliar fungicide to evaluate the effects on milk production and efficiency [37]. The section below will discuss more about the dairy cow and digestive system, but in an economic analysis the total income from milk yield over feed costs in 2015 was $7.35, $7.54, $8.31, and $7.83 for no fungicide application, one application, two applications, or three applications of fungicide, respectively [37]. Therefore, it seems cows fed corn silage from corn with fungicide treatment are more profitable than cows fed corn silage with no treatment.
Corn as a Feed Stuff
In 2010, 43% of U.S. corn was used for livestock and poultry diets, 42% was used for ethanol production, and 11% used for food [53]. Deoxynivalenol can cause acute toxicosis in swine, manifested through intestinal disorders and vomiting [11]. Furthermore, aflatoxin, from the fungus A. flavus and parasiticus, consumed by cattle resulted in weight loss and decreased milk production [54].
Some studies have reported no changes in dry matter intake (DMI) or milk yield when cows were challenged with aflatoxin [55-58]. However, Sulzberger et al. fed 3 different concentrations of a clay product (EcoMix, Ukraine) during an aflatoxin challenge, reported a quadratic treatment effect for DMI and a negative linear treatment effect for milk yield [59]. Kubena et al. reported a reduction in feed consumption that adversely affected feed conversion by broiler chickens exposed to aflatoxin [60]. Additionally, threatening human food security, aflatoxin can be found in the milk of dairy cattle as M1 (Richard) [19] and is toxic to humans. In recent years, crop scientists, microbiologists, and animal nutritionists have sought to develop solutions to reduce the impact of fungi on feed for animals and limit the concentration of toxins in products for human consumption.
Dairy cattle and corn silage
In 2013, India, Brazil, and the former Sudan had the largest population of dairy cattle with 45 million, 23 million, and 15 million, respectively [61]. The United States ranked 8th in population with an estimated 9 million dairy cattle. However, total milk yield was greatest for the United States (81 million tons), followed by India (55 million tons), and China (33 million tons) (FAO, 2015) [61]. Improvements in how dairy cattle are fed may help explain why the United States produced milk more efficiently compared to others.
In the United States, corn silage is one of the most popular forages fed to ruminants. The USDA reported 14% of all corn harvested in 2014 was for corn silage production and 89.4% of dairy operations in the United States included corn silage in the lactating diet [62]. It is important to remember that corn silage is heterogeneous combination of fiber and starch from various parts of the corn plant: including stalks, leaves, cob and kernels. On a dry matter basis, whole plant corn silage is composed of about 57% corn ears, 13% corn leaves, and 31% corn stems [2].
Ensiling corn as corn silage
At time of harvest, dairy producers store and preserve corn material as silage, which can be fed all year. The process of ensiling corn is broken down into four phases with varying lengths of time. The first phase, the aerobic period, is characterized by the reduction of atmospheric O2 within a couple hours postharvest, meanwhile active proteases decompose proteins and carbohydrates to amino acids and soluble carbohydrates. The second phase, the fermentation phase, anaerobic microorganisms compete with one another for nutrients, and in well fermented silages, lactic acid bacteria (LAB) eventually dominate lowering the pH [63]. The third phase, the stable phase, continues with the slow hydrolysis of structural and storage carbohydrates, and if air is properly excluded can last any length of time. The fourth phase, the feed out phase, is where plant material is exposed to O2 causing aerobic organisms to develop [63].
Fungi can also attack the plant material in storage. To limit the growth and colonization, generally, it is recommended to store corn material in dry conditions and as mature crops [19]. The occurrence of fungi in silages usually is the result of poor sealing and poor compaction causing aerobic conditions in the silo, not only causing losses of feed, but also reductions in palatability [63]. Furthermore, visibly molded areas of silages underestimate the amount of fungi within the silage content, as well as the high probability of mycotoxins [63]. More exists than visible by the human eye.
The length of ensiling has been shown to have significant effects on the nutritional content of the feedstuff including: dry matter (Der Bedrosian et al.; Weinberg and Chen),lactic acid concentration (Ferraretto) acetic acid concentration (Der Bedrosian et al.,; Weinberg and Chen; Ferraretto et al.), neutral detergent fiber digestibility (Der Bedrosian et al.; Weinberg and Chen), and concentration of crude protein (Der Bedrosian et al.). One study reported a decrease in digestibility within the first 45 d, but then no difference in digestibility from 45 to 365 days.
Another study reported a continued decrease in NDF digestibility 30-h ensiled for up to 6 mo (Weinberg and Chen). Variability could be due to differences in techniques used, but also differences in sample sizes, as decreasing the sample size increased the digestibility of NDF [64-68].
Dairy diet and intake limitations
Corn silage represents about 40 to 60% of the total mixed ration in the lactating diet. Dry matter intake and energy concentration of corn silage determine the energy intake, and therefore, the cow’s performance [69]. Ruminant forage diets are limited by the amount of fiber within the plant material [70]. Van Soest (1965) reported that NDF is highly correlated with dry matter intake; the higher the concentration of NDF within the diet, the lower the DMI, partially as a result of rumen fill and digestibility [71].
Greater lignification of plant cell walls may increase bulk density of the feedstuff or require greater energy concentration of the diet to meet the nutritional needs [69]. Increased lignin concentration within the plant cell has been thought to be the primary limitation to cell wall digestibility. Intense lignification creates an absolute barrier for rumen bacteria when digesting one plant cell wall and moving to the next cell [72]. Mechanical chopping of plant material and cow chewing assist in creating small tears in the silage allowing rumen microbes and enzymes access to degrade the feedstuff, but even under these conditions, the concentration of lignin within the plant material does not change.
Therefore, techniques to alter the fiber content within the silage may create a more digestible feedstuff for dairy cows and impact milk production. An analysis of 20 experiments reported increasing NDF content (Mean: 36.87 ± 5.81% of DM; Min: 22.30% of DM; Max: 51.60% of DM) of corn silage fed to dairy cattle was negatively associated with lower milk yield (R2=92.1), and lower FCM (R2=88.0) [73]. Furthermore, dry matter intake and milk yield decreased for cows fed diets containing increased concentrations of NDF, ADF, and lignin and decreased fiber digestibility [74]. In an analysis of 162 treatments, DMI and milk yield were 0.7 kg/d and 1.0 kg/d greater, respectively, for cows fed corn silage with high in-vitro digestibility compared to a conventional corn silage [75,76]. Furthermore, in a metaanalysis, 1 percentage unit increase in NDF digestibility, measure in vitro or in situ, resulted in 0.25-kg increase in fat corrected milk [74]. Reducing the amount of fiber present in the cell wall can have positive benefits in terms of production for dairy producers.
Corn silage quality
Nutritionists, producers and veterinarians evaluate corn silage quality when feeding to dairy cattle, as it directly relates to energy intake and milk production. Laboratory procedures and on farm tests allow producers to evaluate the diet quickly and make the necessary updates to the diet. Haerr et al. reported that the soluble fraction of NDF and ADF linearly decreased with fungicide applications (same treatments as in Haerr et al.) [75].
Diseased corn silage
From the previous discussion, it is no surprise fungal disease on corn, ensiled as corn silage can impact the nutritional content within the plant material. Inoculation of Northern Leaf Blight, caused by the fungus Exserohilum turcicum, on corn increased the NDF and ADF concentration 52.6 g/kg of DM and 41.2 g/kg of DM, respectively, compared to non-diseased corn [76]. Fungal colonization on the corn plant causes a competition between the plant and the fungus for nutrients. The plant has many mechanisms (e.g., lignification and leaf shedding) to attempt to hinder the growth of the fungal infestation. These mechanisms may potentially decrease the digestibility of the plant. The fungal infestation itself may also change the chemical composition of the plant in the process of competing for nutrients [77].
The corn was then ensiled as corn silage and fed to sheep. Corn silage from diseased corn resulted in a greater concentration of NDF (499.9 ± 40.1 g/kg of DM) and ADF (263.0 ± 32.5 g/kg of DM) when compared to corn silage from nondiseased corn (392.1 ± 32.1 g/kg of DM and 217.0 ± 30.3 g/kg DM for NDF and ADF, respectively). Dry matter digestibility was less for sheep consuming corn silage from diseased corn (0.665 ± 0.029) compared to control (0.725 ± 0.012), measured using metabolic crates. Yet, dry matter intake was not different for sheep consuming corn silage from diseased corn (34.6 ± 4.1 g/kg of BW0.75 /d) compared to control (40.9 ± 4.1 g/kg of BW0.75 /d) [76].
In another study, corn was ensiled with either no fungi (no rust), a medium concentration (all leaves on the lower half of the plant affected), or a high concentration (all leaves affected) of Southern Rust, caused by the fungus Puccinia polysora, and then, ensiled as corn silage. Increasing the rust infestation from no rust to medium rust to high rust concentration on corn ensiled as corn silage increased the DM concentration, the concentration of NDF (no rust: 44.1% of DM, medium rust: 47.7% of DM, and high rust: 48.5% of DM) and ADF (no rust: 23.1% of DM, medium rust: 25.1% of DM, and high rust: 25.3% of DM), and decreased the in vitro DM true digestibility (no rust: 66.9%, medium rust: 63.2%, and high rust: 60.1%) and in vitro NDF digestibility (no rust: 38.1%, medium rust: 39.8%, and high rust: 36.2%) Additionally, increased rust infestation on corn silage resulted in worse fermentation conditions exhibited by: increased pH (no rust: 3.65, medium rust: 3.71, and high rust: 3.97) and decreased lactate (no rust: 4.99, medium rust: 4.02, and high rust: 2.28%). Aflatoxin was detected in corn silage from corn with a high concentration of Southern Rust at a concentration of 5.20 mg/kg of DM Zearalenone was detected only in corn silage with no concentration of Southern Rust at a concentration of 0.64 mg/kg of DM [78].
Another set of researchers evaluated physically damaging the ears of corn in the field prior to harvest on the production of mycotoxins and fermentation when ensiled as corn silage, to represent insect or hail damage on corn. In the first experiment, physical damage to corn kernels occurred at the milk stage of corn development (R3) slashing a knife through the kernels. Corn from experiment one was ensiled as corn silage for 126 d. Physical damage to the corn ear resulted in an increased concentration of fumonisin B1 (8.50 mg/kg for damaged and 4.00 mg/kg for undamaged DON (3.12 mg/kg for damaged and 0.92 mg/kg for undamaged) but decreased the concentration of zearalenone (1.03 mg/kg for damaged and 0.46 mg/kg for undamaged) in corn silage. Neutral detergent fiber and ADF was not different for corn silage physically damaged (45.0 and 26.8% of DM for NDF and ADF, respectively) compared with undamaged (45.2 and 27.3% of DM for NDF and ADF, respectively). In experiment two, physical damage to the corn kernels occurred either 27 d or 9 d prior to harvest, and was ensiled for 95 d. Damage to corn kernels 27 d prior (29.5% of DM) to harvest resulted in an increased ADF content in corn silage compared to 9 d prior (25.2% of DM) or no damage (25.7% of DM). Corn silage damaged 27 d prior to harvest resulted in an increased concentration of ADF (31.9% of DM) and NDF (48% of DM) when compared to corn silage from nondamaged ears (22.3 and 36.3% of DM for ADF and NDF, respectively) (Teller et al.). Furthermore, corn silage from corn damaged 27 d prior to harvest resulted in an increased concentration of DON (14.77 mg/kg), fumonisin B1 (7.63 mg/ kg), and zearalenone (3.66 mg/kg) when compared with corn silage from undamaged corn kernels (0.18, 1.03, and 0.99 mg/kg for DON, fumonisin B1, and zearalenone, respectively) [79].
Fungicide on corn ensiled as corn silage
Researchers at the University of Wisconsin applied pyraclostrobin on corn and ensiled it as corn silage. Using the MILK 2006 model that predicts the amount of milk to be produced if the corn silage was to be fed to cows, pyraclostrobin application on corn numerically increased projected milk production by 75 lbs milk/ton DM (37 kg milk/ metric ton DM) when compared with control [34].
As previously mentioned, Haerr et al. fed cows corn silage from corn with either one application of foliar fungicide, two applications of foliar fungicide, three applications of foliar fungicide, or no application of foliar fungicide [80]. A decreasing linear relationship was reported for the number of fungicide applications and DMI (23.8, 23.0, 19.5, and 21.3 kg for CON, 1X, 2X, and 3X, respectively) but constant milk production among treatments (34.5, 34.5, 34.2, and 34.3 kg/d, for CON, 1X, 2X, and 3X, respectively) (Haerr et al.) [83]. Therefore, cows fed corn silage from corn treated with foliar fungicide tended to have better-feed conversion milk yield/DMI values (1.46, 1.47, 1.70, and 1.70 kg/kg, for CON, 1X, 2X, and 3X, respectively), 3.5% FCM values (1.47, 1.51, 1.71, and 1.73, for CON, 1X, 2X, and 3X, respectively) and ECM valued (1.43, 1.46, 1.66, and 1.69 for CON, 1X, 2X, and 3X, respectively) [79]. The authors hypothesized that improved feed efficiency occurred because corn silage from corn treated with foliar fungicide application may have had an increased nutritive quality compared to untreated corn silage. Haerr et al. reported that application of fungicide to corn and then ensiled as corn silage (same treatments as in Haerr et al) resulted in higher DM degradable fraction which increased with the number of fungicide applications. It tended to linearly decrease DM solubility. The authors reported that the soluble fraction of NDF and ADF linearly decreased with fungicide applications [75].
Most recently, Kalebich fed cows corn silage from corn with either no, one, two, or three applications of fungicide. Treatments were as follows: control (CON), corn silage with no application of foliar fungicide; treatment 1 (V5), corn silage received one application of pyraclostrobin and fluxapyroxad (PYR+FLUX) foliar fungicide at corn vegetative stage 5 (V5; when the emergence of the fifth leaf is visible); treatment 2 (V5/V8), corn silage received one application of PYR+FLUX at corn stage V5 plus another application of PYR+FLUX at corn stage vegetative stage 8 (V8; when the emergence of the eighth leaf is visible); and treatment 3 (V5/V8/R1), corn silage received one application of PYR+FLUX at corn stage V5, one application of PYR +FLUX at corn stage V8, plus a third application of pyraclostrobin and metconazole (PYR+MET) foliar fungicide at corn stage reproductive stage 1 (R1; when the silks are fully extended). No differences in DMI, milk yield, or feed conversion were reported among treatments. However, cows in V5 compared with cows in V5/V8 tended to produce more 3.5% FCM (32.42 and 28.58 kg/d, respectively), and ECM (31.35 and 27.76 kg/d, respectively). Furthermore, concentration of milk lactose tended to be greater for cows fed corn silage treated with foliar fungicide when compared with CON (4.63, 4.77, 4.76, and 4.72% for CON, V5, V5/V8, and V5/V8/R1, respectively). The authors hypothesized corn silage from corn with fungicide applications may improve the digestibility compared with untreated corn silage [81].
As a follow up study to evaluate the effects of fungicide on fermentation and composition of corn silage, Kalebich et al. prepared 0.9-kg laboratory silos of treatment chopped corn material [82]. Fungicide applications on corn during the summer of 2015 were as follows: control (CON), corn receiving no foliar fungicide application; treatment 1 (V5), where corn received a mixture of pyraclotrobin and fluxapyroxad (PYR+FLUX) foliar fungicide at V5; treatment 2 (V5+R1), where corn received two applications of foliar fungicide, a mixture of PYR+FLUX at V5 and a mixture of pyraclostrobin+metconazole (PYR+MET) foliar fungicide at corn reproductive stage 1 (R1); treatment 3 (R1), in which corn received one application of PYR+MET foliar fungicide at R1. Fungicide treated corn silage had decreased dry matter (335, 319, 315, and 317 g/kg DM for CON, V5, V5+R1, and R1, respectively; P=0.0005), but increased crude protein (81, 85, 82, and 87 g/kg DM for CON, V5, V5+R1, and R1, respectively), water soluble carbohydrates (38, 40, 46, and 52 g/kg DM for CON, V5, V5+R1, and R1, respectively), and lactic acid concentration (46.5, 50.1, 50.9, and 55.0 g/kg DM for CON, V5, V5+R1, and R1, respectively). Corn silage in R1 had a lower lignin concentration (24, 24, 26, and 20 g/kg DM for CON, V5, V5+R1, and R1, respectively), and corn silage in V5 had greater milk kg/MT DM (1511, 1631 1585, and 1576 kg/MT DM for CON, V5, V5+R1, and R1, respectively). Corn silage from corn with fungicide application may enhance the nutritive and fermentative profile for ruminants (Kalebich et al.) [83].
Conclusion
Fungal infections on corn threaten food security by limiting total yield and nutritional quality of crop, ultimately reducing the efficiency of crop production. It is well known that applications of foliar fungicide on corn assist in limiting devastating losses in yield. Less is known about the negatives of making and feeding silage from diseased corn plants. Cows fed corn silage from corn with fungicide treatment improved feed conversion and were more profitable than cows fed corn silage with no fungicide treatment. Corn silage from corn with fungicide application reduced the fiber concentration and improved the nutritive value. Additionally, corn treated with fungicide had improved fermentation process during ensiling due to higher concentrations of lactic acid and sugar when compared with untreated corn. In conclusion, fungicide application seems to be maximized when converted into feed (e.g.; corn silage) and fed to ruminants.
References
- Gilland B (2002) World population and food supply: can food production keep pace with population growth in the next half-century? Food Policy 27:47-63.
- Kuehn CS, Linn JG, Johnson DG, Jung HG, Endres MI (1999) Effect of feeding silages from corn hybrids selected for leafiness or grain to lactating dairy cattle. J Dairy Sci 82: 2746-2755.
- Mueller D, Wise K (2014) Corn disease loss estimates from the United States and Ontario, Canada-2013. Purdue University, Extension publication BP-96-13-W. West Lafayette, IN.
- Malinovsky FG, Fangel JU, Willats WG (2014) The role of the cell wall in plant immunity. Plant cell wall in pathogenesis, parasitism and symbiosis. Front. Plant Sci. 13:38.
- Sexton AC, Howlett BJ (2006) Parallels in fungal pathogenesis on plant and animal hosts. Eukaryot Cell 5: 1941-1949.
- Santiago R, Barros-Rios J, Malvar RA (2013) Impact of cell wall composition on maize resistance to pests and diseases. Int J Mol Sci 14: 6960-6980.
- Calvo AM, Wilson RA, Bok JW,Keller NP (2002) Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66:447-459.
- Nutter FW Jr, Jenco JH (1992) Development of criticial-point yield loss models to estimate yield losesses in corn caused by Cercospora zeae-maydis. (Abstr.) Phytopathology 82:994.
- Ward, JM, Stromberg EL, Nowell DC,Nutter FW Jr (1999) Gray leaf spot: A disease of global importance in maize production. Plant Dis. 83:884-895.
- Shah DA, Dillard HR (2006) Yield loss in sweet corn caused by Puccinia sorghi: A meta-analysis. Plant Dis. 90:1413-1418.
- Miller J D (1995) Fungi and mycotoxins in grain: implications for stored product research. J Stored Prod. Res. 31:1-16.
- Carris LM, Little CR, Stiles CM (2012) Introduction to fungi. The Plant Health Instructor.
- Knogge W (1996) Fungal Infection of Plants. Plant Cell 8: 1711-1722.
- Meng L, Zhang A, Wang F, Han X, Wang D, et al. (2015) Arbuscular mycorrhizal fungi and rhizobium facilitate nitrogen uptake and transfer in soybean/maize intercropping system. Front. Plant. Sci 6: 339.
- Voegele RT, Struck C, Hahn M, Mendgen K (2001) The role of haustoria in sugar supply during infection of broad bean by the rust fungus Uromyces fabae. See comment in PubMed Commons below Proc Natl Acad Sci USA 98: 8133-8138.
- Elmore JM, Coaker G (2011) The role of the plasma membrane H+-ATPase in plant-microbe interactions. Mol Plant 4: 416-427.
- Ariño A, Herrera M, Juan T, Estopañan G, Carramiñana JJ, et al. (2009) Influence of agricultural practices on the contamination of maize by fumonisin mycotoxins. J Food Prot 72: 898-902.
- Freeman BC, Beattie GA (2008) An overview of plant defenses against pathogens and herbivores. Plant Health Instructor.
- Richard JL (2007) Some major mycotoxins and their mycotoxicoses an overview. Int J Food Microbiol 119: 3-10.
- Wise K (2011) Diseases of corn: Northern Corn Leaf Blight in Purdue Extension P. University.
- FAO (2015) Inputs, Pesticide use in Food and Agriculture Organization of the United Nations. FAOSTAT Roma, Italy.
- Balba H (2007) Review of strobilurin fungicide chemicals. J Environ Sci Health B 42: 441-451.
- Vincelli P (2002) Q o I (strobilurin) fungicides: benefits and risks. Plant Health Instructor.
- McKay A, Hagerty G, Follas G, Moore M,Christie M et al. (2011) Succinate dehydrogenase inhibitor(SDHI) fungicide resistance prevention strategy. N. Z. Plant Prot 64: 119-124.
- Lepesheva GI, Waterman MR (2007) Sterol 14alpha-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim Biophys Acta 1770: 467-477.
- Lucas JA, Hawkins NJ, Fraaije BA (2015) The evolution of fungicide resistance. In Advances in Applied Microbiology. Vol. 90. S. Sima and G. Geoffrey Michael, ed. Academic Press.
- Wise K, Mueller D (2011) Are fungicides no longer just for fungi? An analysis of foliar fungicide use in corn. APSnet Features.
- Lipps PE (1987) Gray leaf spot epiphytotic in Ohio corn. Plant Dis. 71: 281.
- Paul PA, Madden LV, Bradley CA, Robertson AE, Munkvold GP, et al. (2011) Meta-analysis of yield response of hybrid field corn to foliar fungicides in the U.S. Corn Belt. Phytopathology 101: 1122-1132.
- Bradley C, Ames K (2010) Effect of foliar fungicides on corn with simulated hail damage. Plant Dis. 94:83-86.
- Nelson KA, Meinhardt C (2011) Foliar boron and pyraclostrobin effects on corn yield. Agron. J. 103:1352-1358.
- MuellerD, Pope R (2009) Corn Field Guide. Iowa State University, Extenstion. and Outreach, Ames, Iowa.
- Wright P, Parker M, Van Tilburg R, Hedderley D (2014) Effect of planting dates and azoxystrobin fungicide application regimes on common rust of maize. N. Z. J. Crop Hortic. Sci 42: 99-110.
- Blonde G, Esker P (2008) The effect of headline foliar fungicide on corn silage yield and quality in forage focus. Madison, WI.
- Yates IE, Bacon CW, Hinton DM (1997) Effects of endophytic infection by Fusarium moniliforme on corn growth and cellular morphology. Plant Dis 81:723-728.
- Kalebich CC, Weatherly ME, Robinson KN, Fellow GM, Murphy MR, et al. (2017) Foliar fungicide (pyraclostrobin) application effects on plant composition of a silage variety corn. Anim. Feed Sci. Tech. Anim. Feed Sci. Tech 225: 38-53.
- Haerr K J (2015) The use of corn treated with various applications of foliar fungicide to increase corn silage quality and performance of Holstein cows in animal sciences. MS Thesis. University of Illinois at Urbana-Champaign.
- Edwards S, Pirgozliev S, Hare SM, Jenkinson P (2001) Quantification of trichothecene-producing Fusarium species in harvested grain by competitive PCR to determine efficacies of fungicides against Fusarium head blight of winter wheat. Appl. Environ. Microbiol 67: 1575-1580.
- Magan N, Hope R, Colleate A, Baxter E (2002) Relationship between growth and mycotoxin production by Fusarium species, biocides and environment. Mycotoxins in Plant Disease. Springer. Beford, UK.
- Schmidt-Heydt M, Stoll D, Geisen R (2013) Fungicides effectively used for growth inhibition of several fungi could induce mycotoxin biosynthesis in toxigenic species. International Journal of Food Microbiology 166: 407-412.
- Braunberg RC, Barton CN, Gantt OO, Friedman L (1994) Interaction of citrinin and ochratoxin A. Nat Toxins 2: 124-131.
- Schmidt-Heydt M, Graf E, Stoll D, Geisen R (2012) The biosynthesis of ochratoxin A by Penicillium as one mechanism for adaptation to NaCl rich foods. Food Microbiol 29: 233-241.
- Alonso VA, Pereyra CM, Keller LA, Dalcero AM, Rosa CA, et al. (2013) Fungi and mycotoxins in silage: an overview. J Appl Microbiol 115: 637-643.
- Agrios GN (1997) Plant pathology (4th edn) Academic Press, San Diego, CA
- Whitlock LA, Wistuba TJ, Seifers MK, Pope RV, Bolsen KK (2000) Effect of level of surface-spoiled silage on the nutritive value of corn silage diets. J. Dairy Sci 46.
- Gerlach K, Weiß K, Roß F, Buscher W, Sudekum KH (2013) Changes in maize silage fermentation products during aerobic deterioration and its impact on feed intake by goats. Agric. Food Sci. 22: 168-181.
- Driehuis F, Spanjer MC, Scholten JM, te Giffel MC (2008) Occurrence of mycotoxins in feedstuffs of dairy cows and estimation of total dietary intakes. J Dairy Sci 91: 4261-4271.
- Adesogan AT (2006) Mycotoxins in ensiled forages. Pages 44-51 in Key Silage Management Topics. R. Charley, ed. Lallemand Animal Nutrition North America, Milwaukee, WI.
- Doerr JA (2010) A little fresh air: Fungal toxins and silage. California Alfalfa and Forage Symposium and Corn/Cereal Silage Mini-Symposium, Visalia, CA. University of California, Davis, CA.
- Whitlow LW, Hagler WM Jr (2008) Mold and mycotoxin issues in dairy cattle: Effects, prevention and treatment. Advances in Dairy Technology, Vol. 20: Proc. Western Canadian Dairy Seminar, Red Deer, AB, Canada. University of Alberta, Edmonton, AB Canada.
- Korosteleva SN, Smith TK, Boermans HJ (2009) Effects of feed naturally contaminated with Fusarium mycotoxins on metabolism and immunity of dairy cows. J Dairy Sci 92: 1585-1593.
- Charmley E, Trenholm HL, Thompson BK, Vudathala D, Nicholson JW, et al. (1993) Influence of level of deoxynivalenol in the diet of dairy cows on feed intake, milk production, and its composition. J Dairy Sci 76: 3580-3587.
- NASS (2010) US. Livestock Industry and Crop Statisitcs. Natl. Ag. Statistics Ser, Washington DC.
- Miller DM, Wilson DM (1994) Veterinary diseases related to aflatoxin. The Toxicology of Alfatoxins D. L. Eaton, Groopman, J.D, edn. Academic Press Inc., New York.
- Battacone G, Nudda A, Palomba M, Mazzette A, Pulina G (2009) The transfer of aflatoxin M1 in milk of ewes fed diet naturally contaminated by aflatoxins and effect of inclusion of dried yeast culture in the diet. J Dairy Sci. 92: 4997-5004.
- Queiroz OC, Han JH, Staples CR, Adesogan AT (2012) Effect of adding a mycotoxin-sequestering agent on milk aflatoxin Mâ‚ÃÆââ¬Å¡Ãâàconcentration and the performance and immune response of dairy cattle fed an aflatoxin Bâ‚ contaminated diet. J Dairy Sci 95: 5901-5908.
- Maki CR, Thomas AD, Elmore SE, Romoser AA, Harvey RB, et al. (2016) Effects of calcium montmorillonite clay and aflatoxin exposure on dry matter intake, milk production, and milk composition. J Dairy Sci 99: 1039-1046.
- Maki CR, Monteiro APA, Elmore SE, Tao S, Bernard JK, et al. (2016) Calcium montmorillonite clay in dairy feed reduces aflatoxin concentrations in milk without interfering with milk quality, composition or yield. Anim. Feed Sci. Technol. 214: 130-135.
- Sulzberger SA, Melnichenko S, Cardoso FC (2017) Effects of clay after an aflatoxin challenge on aflatoxin clearance, milk production, and metabolism of Holstein cows. J Dairy Sci 100: 1856-1869.
- Kubena LF, Harvey RB, Bailey RH, Buckley SA, Rottinghaus GE (1998) Effects of a hydrated sodium calcium alluminosillicate (T-Bind) on mycotoxicosis in young broiler chickens. Poult. Sci. 77: 1502-1509.
- FAO (2015) Live Animals. in Food and Agriculture Organization of the United Nations. FAOSTAT Roma, Italy.
- USDA (2014) Dairy 2014. in Dairy Cattle Management Practices in United States. Washington, DC.
- Pahlow G, Muck R, Driehuis F, Oude Elferink SJWH, Spoelstra SF (2003) Microbiology of Ensiling. Silage Science and Technology R. E. M. D. R. Buxton, and J. H. Harrison ed. American Society of Agronomy, Inc., Crop Science Society of America, Inc., and Soil Science Society of American, Inc. Madison, WI.
- Der Bedrosian MC, Nestor KE Jr, Kung L Jr (2012) The effects of hybrid, maturity, and length of storage on the composition and nutritive value of corn silage. J Dairy Sci 95: 5115-5126.
- Weinberg Z, Chen Y (2013) Effects of storage period on the composition of whole crop wheat and corn silages. Anim. Feed Sci. Technol. 185:196-200.
- Ferraretto LF, Shaver RD2 (2015) Effects of whole-plant corn silage hybrid type on intake, digestion, ruminal fermentation, and lactation performance by dairy cows through a meta-analysis. J Dairy Sci 98: 2662-2675.
- Ferraretto L, Shaver R, Massie S, Singo R, Taysom D, et al. (2015) Effect of ensiling time and hybrid type on fermentation profile, nitrogen fractions, and ruminal in vitro starch and neutral detergent fiber digestibility in whole-plant corn silage. Prof. Anim. Sci. 31: 146-152.
- Malebana I, Cherney D, Parsons D, Cox W (2015) Corn silage analysis as influenced by sample size collected. Anim. Feed Sci. Technol 210: 17-25.
- Allen MS, Coors JG, Roth GW (2003) Corn Silage. Silage Science and Technology. D. Buxton, R. Muck, and J. Harrison ed. American Society of Agronomy, Crop Science Society of America, Soil Society of American. Madison, WI.
- Van Soest PJ (1994) Nutritional Ecology of Ruminant. Cornell University Press, Ithaca, NY.
- Van Soest PJ (1965) Symposium on factors influencing the voluntary intake of herbage by ruminants: voluntary intake in relation to chemical composition and digestibility. J. Anim. Sci 834-843.
- Jung HG (2012) Forage digestibility: The intersection of cell wall lignification and plant tissue anatomy. Proceedings of the 23rd annual Florida ruminant nutrition symposium. University of Florida: Gainesville, FL.
- Briceno JV, Van Horn HH, Harris B Jr, Wilcox CJ (1987) Effects of neutral detergent fiber and roughage source on dry matter intake and milk yield and composition of dairy cows. J Dairy Sci 70: 298-308.
- Oba M, Allen MS (1999) Evaluation of the importance of the digestibility of neutral detergent fiber from forage: effects on dry matter intake and milk yield of dairy cows. J Dairy Sci 82: 589-596.
- Haerr KJ, Pineda A, Lopes NM, Weems JD, Bradley CA, et al. (2016) Effects of corn treated with foliar fungicide on in situ corn silage degradability in Holstein cows. Anim. Feed Sci. Tech 222: 149-157
- Wang P, Souma K, Kobayashi Y, Iwabuchi K, Sato C, et al. (2010) Influences of Northern Leaf Blight on corn silage fermentation quality, nutritive value and feed intake by sheep. Anim Sci J 81: 487-493.
- Venancio WS, Rodrigues MAT, Begliomini E, De Souza NL (2009) Physiological effects of strobilurin fungicides on plants. Ci. Exatas e da Terra Ci Agr Eng 9: 59–68.
- Queiroz OC, Kim SC, Adesogan AT (2012) Effect of treatment with a mixture of bacteria and fibrolytic enzymes on the quality and safety of corn silage infested with different levels of rust. J Dairy Sci 95: 5285-5291.
- Teller R, Schmidt R, Whitlow L, Kung L (2012) Effect of physical damage to ears of corn before harvest and treatment with various additives on the concentration of mycotoxins, silage fermentation, and aerobic stability of corn silage. J. Dairy Sci. 95: 1428-1436.
- Haerr KJ, Lopes NM, Pereira MN, Fellows GM, Cardoso FC (2015) Corn silage from corn treated with foliar fungicide and performance of Holstein cows. J Dairy Sci 98: 8962-8972.
- Kalebich CC (2016) From field to rumen: Foliar fungicide application on corn and its effects on the corn plant, corn silage, and Holstein cow performance. MS Thesis. University of Illinois at Urbana-Champaign.
- Kalebich CC, Weatherly ME, Robinson KN, Fellow GM, Murphy MR, et al. (2017) Foliar fungicide (pyraclostrobin) application on corn and its effects on corn silage composition. Anm. Feed Sci. Tech. In press.
- MortonV T, Staub T (2008) A short history of fungicides. APSnet Features.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences