Factors Affecting Nitrite Concentrations in Cat Food
Jun Kobayashi, Keiichi Ikeda, Hideo Sugiyama
DOI10.21767/2572-5459.100028
Jun Kobayashi1*, Keiichi Ikeda2 and Hideo Sugiyama3
1Faculty of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan
2Faculty of Pharmaceutical Sciences, Hokuriku University, Ishikawa, Japan
3Graduate School of Health Sciences, Matsumoto University, Nagano, Japan
- Corresponding Author:
- Jun Kobayashi
School of Veterinary Nursing and Technology
Faculty of Veterinary Medicine
Nippon Veterinary and Life Science University
1-7-1 Kyonan-cho, Musashino, Tokyo 180-8602, Japana
Tel: +81422314151
Fax: +81422332094
E-mail: junkoba@nvlu.ac.jp
Received Date: May 03, 2017; Accepted Date: May 31, 2017; Published Date: June 05, 2017
Citation: Kobayashi J, Ikeda K, Sugiyama H. Factors Affecting Nitrite Concentrations in Cat Food. J Anim Res Nutr 2017, 2:8. doi:10.21767/2572-5459.100028
Copyright: © 2017 Kobayashi J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Context: Regulations for pet food production and additives in Japan are defined by the Law for Ensuring the Safety of Pet Food, but are not as detailed as regulations concerning food for human consumption.
Objective: Nitrite is a food additive used to improve color and taste and to control botulinum. We examined the levels of sodium nitrite in 33 samples of commercially available wet-type cat foods.
Design: We measured nitrite levels in the samples, and stratified the findings by ingredient. We also estimated the upper limit concentration for sodium nitrite in cat foods compared with the standard concentration defined by current Japanese law.
Results: Concentrations ranged from 0.05 to 6.14 mg/kg. Nitrite levels were highest in beef formulations is the upper limit concentration calculated was considerably lower than the concentration mandated by law, and eight of the samples measured exceeded the upper limit.
Conclusions: It may be necessary to set stricter limits for nitrite contents in cat
Keywords
Cat food; Nitrite; Concentration change
Introduction
Recognition of pets as family members is increasing among dog and cat owners, coupled with a desire for safe pet food products. In Japan, regulations for pet food production are defined by the Law for Ensuring the Safety of Pet Food (also termed the Pet Food Safety Law), but pet food is less regulated than food for human consumption. Regulations regarding pet food additives are not fully developed.
Nitrite is a commonly used additive in pet food, whose regulation began in 2015. In foods for human consumption, the Standard Methods of Analysis for Hygienic Chemistry stipulate a concentration of 5–70 ppm as the maximum residual amount of nitrites allowed [1]. For pet food, the Pet Food Safety Law caps permissible nitrite levels at 100 ppm (as sodium nitrite for 10% moisture content). However, the Association of American Feed Control Officials (AAFCO) has published a recommended standard of ≤ 20 ppm [2].
In this study, we measured nitrite levels in commercially available wet-type cat foods. Based on the results, we investigated whether nitrites were labeled correctly on the packaging, and whether different ingredients influenced nitrite concentrations in the food. Furthermore, we compared nitrite concentrations in different batches of the same food, to determine whether they varied depending on manufacturing date and place. Finally, we performed an independent estimate of the upper limit concentration of sodium nitrite appropriate for use in cat food.
Materials and Methods
Samples
Thirty-three commercially available wet foods classified as complete-nutrition food for cats were obtained in April and August 2013 and used in the study (Table 1). The samples were sealed in small bags and stored in a freezer at −30°C, then defrosted prior to analysis.
| Sample No. | Manufacturer | Country of origin | Content (meat and fish/vegetable) | Package form | Nitrite addition* |
|---|---|---|---|---|---|
| 1 | A | USA | chicken, pork, turkey/rice, spinach | can | |
| 2 | A | USA | chicken, pork, shellfish/carrot, spinach, tomato | can | |
| 3 | A | USA | chicken, pork, shellfish | can | |
| 4 | A | USA | chicken, pork | can | |
| 5 | B | USA | chicken, pork | can | |
| 6 | B | USA | chicken, pork, shellfish | can | |
| 7 | B | USA | chicken, pork, shellfish/corn | can | |
| 8 | B | USA | chicken, pork | can | |
| 9 | B | USA | chicken, pork, turkey/rice | can | |
| 10 | C | USA | beef, pork | can | |
| 11 | D | Austria | chicken, pork, shellfish | pouch | |
| 12 | D | Austria | chicken, pork, shellfish | pouch | |
| 13 | E | Thailand | Shellfish | pouch | |
| 14 | E | Thailand | Shellfish | pouch | |
| 15 | E | Thailand | chicken, shellfish | pouch | |
| 16 | E | Thailand | Shellfish | pouch | |
| 17 | F | Thailand | beef, chicken, shellfish | pouch | ÃÆÃÂÃâþ |
| 18 | F | Thailand | beef, chicken, shellfish | pouch | ÃÆÃÂÃâþ |
| 19 | F | Thailand | beef, chicken, shellfish | pouch | ÃÆÃÂÃâþ |
| 20 | F | Thailand | beef, chicken, shellfish | pouch | ÃÆÃÂÃâþ |
| 21 | F | Thailand | beef, chicken, shellfish | pouch | ÃÆÃÂÃâþ |
| 22 | F | Thailand | beef, chicken | pouch | ÃÆÃÂÃâþ |
| 23 | F | Thailand | beef, chicken, turkey | pouch | ÃÆÃÂÃâþ |
| 24 | G | Thailand | chicken, egg (powder), shellfish | pouch | |
| 25 | G | Thailand | chicken, egg (powder), shellfish | pouch | |
| 26 | G | Thailand | chicken, egg (powder), shellfish | pouch | |
| 27 | H | Japan | chicken, egg (powder), shellfish/rice, vegetable (powder) | can | |
| 28 | H | Japan | chicken, egg (powder), shellfish/rice, vegetable (powder) | can | |
| 29 | I | Japan | cheese, chicken, shellfish | can | |
| 30 | I | Japan | chicken, egg, shellfish | can | |
| 31 | J | Japan | beef, chicken/carrot | can | ÃÆÃÂÃâþ |
| 32 | J | Japan | Shellfish | can | |
| 33 | J | Japan | chicken, shellfish | can |
Table 1: Cat foods tested for nitrites in the study
Multiple batches with varying lot numbers of samples 1,2, and 13-18 were acquired in April and October 2013, to determine whether nitrite concentrations differed between production batches. Samples purchased in October were not frozen and defrosted prior to analysis.
Reagents
Sulfanilamide solution was prepared by dissolving 0.5 g of sulfanilamide (special grade, Wako Pure Chemical Industries, Osaka, Japan) in 100 ml of hydrochloric acid (special grade, Wako Pure Chemical Industries). A naphthylethylenediamine solution (Griess reagent) was prepared by dissolving 0.12 g of N- (1-naphthyl) ethylenediamine hydrochloride (special grade, Wako Pure Chemical Industries) in 100 ml of water and stored away from light. The nitrite standard stock solution was prepared by dissolving 0.023 g of sodium nitrite (special grade, Wako Pure Chemical Industries) in 50 ml of water, and this was further diluted 500-fold with water to obtain the nitrite standard solution (0.6 μg/ml nitrite). To prepare an ammonium acetate solution, 10 g of ammonium acetate (special grade, Wako Pure Chemical Industries) were added to 100 ml of water. A sodium hydroxide solution was prepared by adding 2 g of sodium hydroxide (special grade, Nacalai Tesque, Kyoto, Japan) to 100 ml of water. A zinc sulfate solution was prepared by adding 21.4 g of zinc sulfate heptahydrate (special grade, Nacalai Tesque) to 100 ml of water. Water used in all reagents and experiments was purified using a Simplicity purifier (Merck Millipore, Billerica, MA, USA; 18.2 MΩ · cm).
Apparatus
The following instruments were used in the experiments: A PA 64C precision balance (Ohaus, Tokyo); a thermostatic bath (Thermominder SDminiN, Taitec, Saitama, Japan); a microcooled centrifuge, model 3780 (Kubota, Tokyo, Japan); a UVmini-1240 spectrophotometer (Shimadzu, Kyoto, Japan).
Measurement of nitrite concentrations
We used the Standard Methods of Analysis for Hygienic Chemistry, 2015 edition [3], with the pretreatment scale set to 1/4 and the measurement scale to 1/10. Measurements were made over three times for each sample.
Approximately 2.5 g of sample were weighed, added to a small amount of warm water (80°C), and homogenized using a mortar and pestle, then transferred to a 50-ml tube. The equipment used for homogenizing was rinsed lightly with warm water and the liquid was added to the tube, to make 30 ml. Next, 2.5 ml each of sodium hydroxide solution and zinc sulfate solution were added, and the tube was shaken well and heated in a water bath set to 80°C for 20 min. The tube was then cooled to room temperature, and 5 ml of ammonium acetate solution were added, followed by enough water for a final volume of 50 ml. After centrifugation at 3,000 rpm for 5 min at 4°C, the supernatant was filtered through filter paper (Advantec Toyo, Tokyo, Japan) to obtain a colorless test solution.
Next, 0.1 ml of sulfanilamide solution and 0.1 ml of naphthylethylenediamine solution were added to a 2-ml aliquot of each test solution. The absorbance (A1) of the solution was measured at 540 nm. To determine the turbidity of the filtrate, we measured the absorbance of the filtrate, mixed with water (A0). We used the absorbance obtained from the calculation (A1 - A0) to obtain the nitrite concentration from a calibration curve. The calibration curve was prepared by diluting the nitrite standard solution with water and analyzing 2 ml each of solutions corresponding to 0, 0.12, 0.24, 0.36, 0.48, and 0.6 μg/ml nitrite in the same manner as the test solution.
Results are presented as mean ± standard deviation (in the text and tables) or mean ± standard error (in the figures). Data were statistically analyzed using Student’s t-test. p<0.05 was considered to represent a statistically significant difference.
Results and Discussion
Table 2 displays the nitrite concentrations measured in cat food samples. Concentrations ranged from 0.05 to 6.14 mg/kg. It has been reported that as much as 4 mg/kg of nitrite may be detected in human meat products even when nitrite is not added [4]. However, in the wet cat food analyzed in this study, only low concentrations were detected. Even in the sample enriched with sodium nitrite, only one aliquot contained more than 4 mg/kg.
| Sample No. | Nitrite concentration (mg/kg)* |
|---|---|
| 1 | 0.637 ± 0.197 |
| 2 | 0.410 ± 0.167 |
| 3 | 0.370 ± 0.147 |
| 4 | 0.390 ± 0.118 |
| 5 | <0.05 |
| 6 | <0.05 |
| 7 | <0.05 |
| 8 | <0.05 |
| 9 | <0.05 |
| 10 | 0.123 ± 0.061 |
| 11 | <0.05 |
| 12 | <0.05 |
| 13 | 0.389 ± 0.088 |
| 14 | 0.421 ± 0.002 |
| 15 | 0.604 ± 0.300 |
| 16 | 0.580 ± 0.105 |
| 17 | 1.186 ± 0.151 |
| 18 | 1.192 ± 0.010 |
| 19 | 1.194 ± 0.231 |
| 20 | 1.233 ± 0.253 |
| 21 | 1.179 ± 0.192 |
| 22 | 1.769 ± 0.556 |
| 23 | 1.676 ± 0.474 |
| 24 | <0.05 |
| 25 | <0.05 |
| 26 | <0.05 |
| 27 | 0.065 ± 0.057 |
| 28 | 0.059 ± 0.033 |
| 29 | <0.05 |
| 30 | 0.077 ± 0.031 |
| 31 | 6.141 ± 0.616 |
| 32 | 0.077 ± 0.046 |
| 33 | 0.096 ± 0.040 |
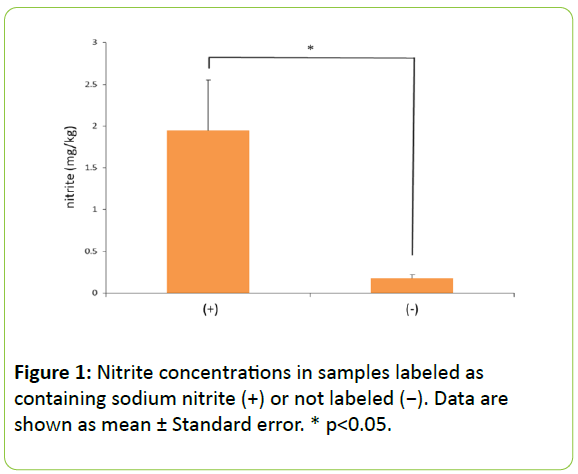
Differences in nitrite concentrations in cat food labeled as containing sodium nitrite: Figure 1 shows a comparison between samples from cat food labeled as containing sodium nitrite and samples from the other foods tested. In samples from foods labeled as containing sodium nitrite, the nitrite concentrations ranged from 1.18 to 6.14 mg/kg; the other samples contained 0.05-0.64 mg/kg. This difference was statistically significant (p<0.05). According to the Pet Food Safety Law, all ingredients used, including additives, must be indicated on the label in principle, and even if sodium nitrite is added, it should be indicated as an ingredient. We found that samples labeled as containing sodium nitrite did indeed contain higher concentrations, in line with the mandates of the Pet Food Safety Law.
Some traces of nitrite were detected even in samples not labeled for sodium nitrite. It is possible that the ingredients used contained nitrites prior to manufacturing. Nitrite has been detected in raw meat and vegetables [4,5], even in trace amounts. It is possible that sodium nitrite was already added to meat before processing. It is also possible that the food was contaminated at the time of manufacture. Verifying this hypothesis would not be feasible. Alternately, it is possible that nitric acid was generated during the manufacturing process. According to Iijima et al. nitrogen in the air can be oxidized by combustion, and the generated nitrite ions may adhere to product and package surfaces, even when a coloring agent is not added to meat products. Consequently, nitrites can be detected in some meats. If canned and pouched foods are later heated for the purpose of sterilization, it is unlikely that this phenomenon would occur. When containers are sealed, the odds of oxidation are considered low. However, manufacturers of pet food may not go to such lengths to prevent the generation of nitrite ions [4].
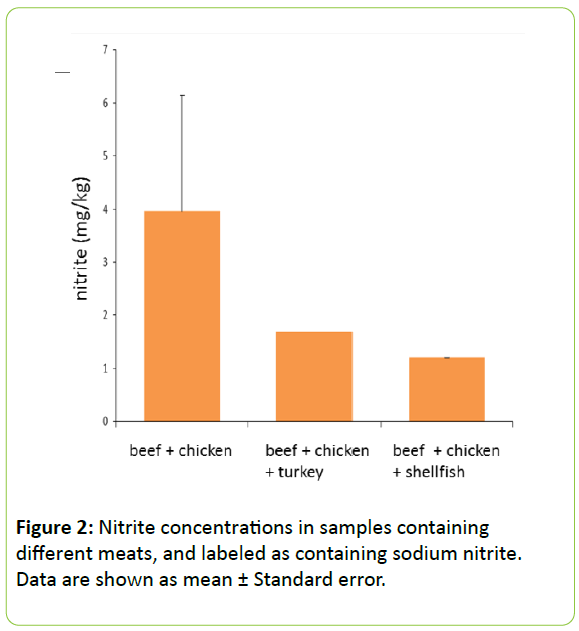
Nitrite concentrations in different meats: We evaluated the differences in nitrite concentrations in different meats, and stratified the analysis depending on whether the samples were labeled as containing sodium nitrite or not. Chicken and beef were listed ingredients in all samples labeled with sodium nitrite (Figure 2). Nitrite concentrations were higher in samples containing only chicken and beef as meats. One company, which uses beef as the main ingredient, may have inflated the mean value. In all the samples marked as containing sodium nitrite, the stated purpose was coloring; however, nitrite reacts with myoglobin in muscle tissue. The red color of raw meat is due to the presence of myoglobin, and becomes a deeper red when myoglobin content is high. Myoglobin content in muscle has been reported to be 0.4%–1.0% in adult cattle, 0.05%–0.3% in swine, and 0.01%–0.15% in poultry, indicating that beef would display as a deeper red than other meats [6]. This suggests that when sodium nitrite is added as a coloring agent, the amount required for beef is greater than the amount required for pork or poultry. To ascertain whether nitrite contents correlate with beef contents, samples of beef-only pet food would be required, which use sodium nitrite strictly as a coloring agent.
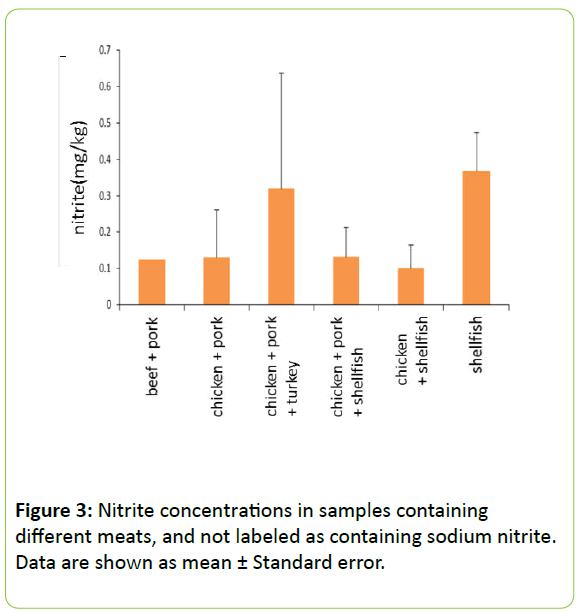
We also compared nitrite concentrations in pet food formulations not labeled as containing sodium nitrite (Figure 3). Samples from foods containing a mix of chicken, pork, and turkey and foods containing only shellfish displayed higher levels of nitrites.
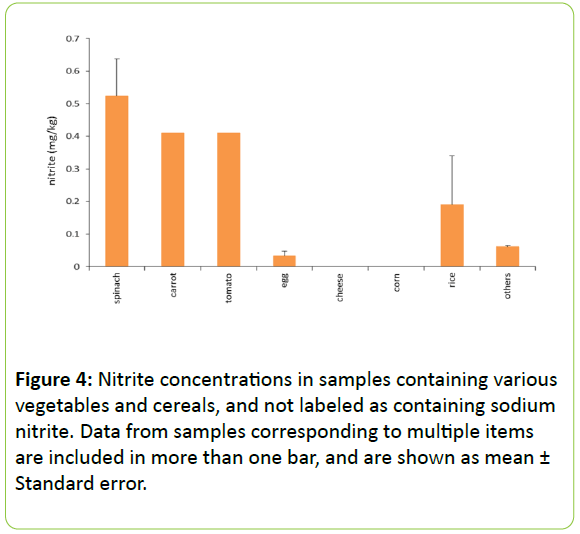
Nitrite concentrations in foods containing various vegetables and cereals: Figure 4 displays the levels of nitrites in foods not labeled as containing nitrites, stratified by vegetable and cereal contents. As most foods contained more than one type of vegetable or cereal, data from some samples appear in more than one bar. Concentrations were relatively high in samples containing spinach, carrots, and tomatoes. A range of 0.2–0.5 mg/kg has been detected in these vegetables [5]. Although 0.3 mg/kg nitrite has been reported in maize [5], nitrite was not detected in the samples labeled as containing corn. The vegetable content may have varied between samples from different pet foods, and the nitrate content in vegetables must also be considered. Many vegetables, especially leafy greens such as spinach, are known to contain nitrates, and these can be reduced to nitrite by nitrate-reducing bacteria in meat and the environment. Therefore, the nitrate contained in the vegetables may be reduced to nitrite during the manufacturing process prior to sterilization. Few foods labeled as containing sodium nitrite also contained vegetables, so we could not compare the two groups.
Nitrite concentrations in canned vs. pouched foods: Figure 5 shows a comparison of nitrite concentrations in canned vs. pouched foods, none of which were labeled as containing sodium nitrite. Foods packed in pouches contained slightly higher concentrations of nitrites. However, there are a few differences in the manufacturing process of pouched and canned foods, but no difference in the shelf life of the products. We consider the result to have occurred by chance, as few samples were tested.
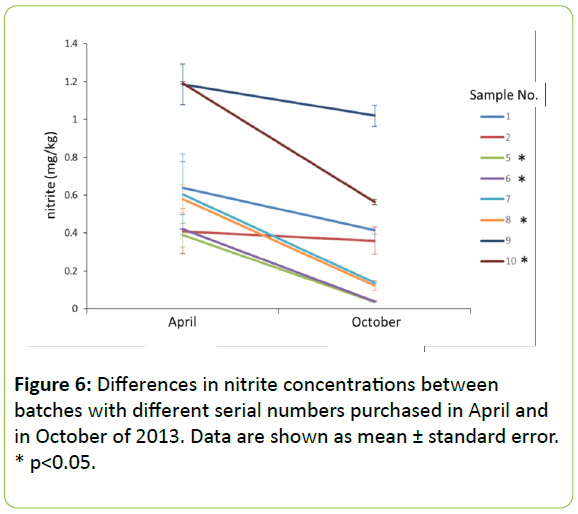
Comparison of nitrite concentrations in samples from different production lots: Figure 6 displays the differences in nitrite concentrations in foods tested on separate occasions; to compare multiple batches (samples 1, 2, and 13–18). Nitrite concentrations in all samples were lower in foods purchased in October 2013 than in foods purchased in April 2013. The degree of decrease in nitrite concentration varied by sample. Some levels did not vary by much, while others decreased to approximately half or even 10%. Concentrations in samples 13, 14, 16, and 18 were significantly higher in April than in October (p<0.05). It is possible that nitrite concentrations in ingredients vary by season. The concentration of nitrate in vegetables is thought to vary depending on harvest time, soil environment, and cultivation conditions, even for the same plant species, and these variations may be similar for nitrite. The concentrations of nitrite in fish and meat may also fluctuate. Another possibility is that the transport method may account for the variations observed. Samples 1, 2, and 13–18 were manufactured in the United States and Thailand, and then shipped to Japan. The transportation period would vary depending on the route and ports used, but is approximately 1 to 2 weeks from Thailand and 1 month from the United States. It is assumed that pet foods would be stored in shipping containers with minimal air conditioning. The stability of a sodium nitrite aqueous solution has already been reported, and no change in concentration was observed even when maintained at 60°C for 120 days [7]. It is therefore unlikely that nitrite concentrations were affected only by the ambient temperature during shipping. Another consideration is pH. The nitrite ion is stable in its alkaline state, but is known to become unstable under more acidic conditions. The half-lives of nitrite ion at 37°C are 9, 24, 739 h, and ∞ at pH 2.6, 4.1, 6.5, and 8.0, respectively [7]. It appears that a low pH would not be detectable in the samples tested, but may register at 6.5. In that case, nitrite concentrations would take approximately 739 h to halve at 37°C. However, it is expected that the nitrite ion reduction rate will rise at higher temperatures. If it is assumed that the sample used is neutral or weakly acidic and the samples purchased in April were transported in winter, while samples purchased in October were transported in summer, it is plausible that the samples purchased in October would exhibit lower concentrations.
Based on the above results, we hypothesize that the nitrite concentration in a given brand of pet food would differ by production date and location. Future studies should measure these variations.
Calculation of the upper limit of sodium nitrite in wet cat food: In Japan, regulation of sodium nitrite in pet food began in 2015, and permissible levels vary from those in food for human consumption. Therefore, based on the allowable daily intake (ADI) of sodium nitrite, we attempted to calculate the concentration to set for cat food.
The ADI of sodium nitrite is 0.07 mg/kg body weight/day. Cats weighing 1 kg can therefore ingest up to 0.07 mg nitrite per day. For example, if we consider wet food sample no. 31 (moisture content approximately 85%, with a recommended daily portion of about 100 g/kg body weight), then the ADI for that food would be:
0.07 mg/100 g=0.7 ppm
To comply with the ingredient specifications of the Pet Food Safety Law, if the moisture content is corrected to 10%, the nitrite concentration that can remain in the cat food is
0.7 ppm × (100 − 10)%/(100−85)%=4.2 ppm
Based on this value, eight of the 33 samples we tested would exceed the concentration standard. Furthermore, when converted into sodium nitrite,
4.2 × (molecular weight of sodium nitrite)/(molecular weight of the nitrite ion)=6.3 ppm
This value is much lower than the current legal regulation standard of less than 100 ppm.
These values were calculated only from one company’s pet food, and the amount and moisture content of the food vary depending on the product. In addition, as older cats have been shown to accumulate nitrite in the intestine, it is possible that the metabolic rate of nitrite may differ depending on age [8]. Furthermore, the metabolic pathway and efficiency of nitrite biotransformation may differ between cats and humans. It is therefore difficult to calculate the ADI as simply as was described above when setting regulatory guidelines. Nevertheless, we think that in the case of cats, which often eat only one pet food daily, it is necessary to set a recommended concentration of sodium nitrite that is stricter than the standard for foods for human consumption. Converted concentrations from the 33 samples are displayed in Table 3.
| Sample No. | Nitrite at the moisture of 10% (mg/kg)*1 | Sodium nitrite (mg/kg)*1 |
|---|---|---|
| 1 | 3.822 | 5.733 |
| 2 | 2.46 | 3.69 |
| 3 | 2.22 | 3.33 |
| 4 | 2.34 | 3.51 |
| 5 | -*2 | - |
| 6 | - | - |
| 7 | - | - |
| 8 | - | - |
| 9 | - | - |
| 10 | 0.738 | 1.107 |
| 11 | - | - |
| 12 | - | - |
| 13 | 2.334 | 3.501 |
| 14 | 2.526 | 3.789 |
| 15 | 3.624 | 5.436 |
| 16 | 3.48 | 5.22 |
| 17 | 7.116 | 10.67 |
| 18 | 7.152 | 10.73 |
| 19 | 7.164 | 10.75 |
| 20 | 7.398 | 11.1 |
| 21 | 7.074 | 10.61 |
| 22 | 10.61 | 15.92 |
| 23 | 10.06 | 15.08 |
| 24 | - | - |
| 25 | - | - |
| 26 | - | - |
| 27 | 0.39 | 0.585 |
| 28 | 0.354 | 0.531 |
| 29 | - | - |
| 30 | 0.462 | 0.693 |
| 31 | 36.85 | 55.27 |
| 32 | 0.462 | 0.693 |
| 33 | 0.576 | 0.864 |
*2Not calculated because the value was below the limit of detection.
Table 3: Converted concentrations.
Conclusion
In this study, we measured nitrite concentrations in wet-type cat food and compared different formulations. We also attempted to investigate concentration variations between different production lots of the same foods, and estimated the upper limit of exposure. Within the scope of this examination, no sampled foods contained concentrations of nitrite that exceeded regulated standards. However, we cannot conclude definitively that these levels are safe. Unlike humans, cats often consume the same food day after day, and their gastrointestinal metabolic processes may differ from human metabolic processes. Therefore, it may be necessary to set stricter guidelines.
When a compound containing nitrites is added to meat, nitrite reacts with myoglobin in muscle tissue to produce nitrosomyoglobin, which is a red dye specific to meat products. This dye becomes more stable after heating [8]. Based on this reaction, the use of sodium nitrite as a color developer for processed meat products is permitted in Japan. Nitrites can also improve the flavor of meat, reduce the oxidation-inhibiting effect of lipids, and exert antibacterial action against Clostridium botulinum [9,10]. However, intake of large quantities of nitrites causes methemoglobinemia and may lower the blood’s oxygentransporting capacity [8,10]. The minimum lethal dose of sodium nitrite has been reported as 0.18 g for infants and 2.5 g for adults [10], and the LD50 of orally administered potassium nitrite has been reported as 220 mg/kg in mice and 85 mg/kg in rats [8]. Moreover, nitrites can react with a secondary amine under acidic conditions similar to those in the stomach to generate N-nitroso compounds, which are potent carcinogens. When nitrite compounds and secondary amines were coadministered to experimental animals, N-nitroso compounds were generated in the stomach and gastrointestinal tract and experimentally shown to be carcinogenic [8].
The addition of sodium nitrite to cat food alters its color, improves meat flavor, inhibits lipid oxidation, and controls botulinum. The color-development effect is probably not necessary for cats. Nevertheless, the sodium nitrite is added for owners concerned with the color of the food. The meatflavoring effect has been shown to improve people’s perception of the meat’s taste, but again, whether the same applies to cats is unknown. As for the lipid oxidation-inhibiting effect, many other antioxidant substances are available, so sodium nitrite is not essential. Therefore, the botulinum-controlling effect of sodium nitrite remains the sole pertinent reason for adding it to cat food. In the United States, the permissible amount of sodium nitrite in meat products other than bacon is 156 mg/kg, of which approximately 25 mg/kg are used for coloring and flavoring the products [11]. Much of the rest is said to be used for controlling botulinum, which requires ≥ 100 mg/kg of sodium nitrite. When sodium nitrite is added to sausage at 100 mg/kg, the remaining amount of nitrite ion is reported to be approximately 15 mg/kg [12]. In this experiment, there were no samples with nitrite concentrations ≥ 10 mg/kg. Although it is not possible to simply compare sausages and pet foods, no cat food marketed in Japan is enriched with sodium nitrite for the purpose of suppressing botulinum. If controlling botulinum is not the stated purpose, there is no reason to add sodium nitrite to cat food. We believe that the addition of sodium nitrite to cat food is unnecessary.
References
- Tanaka A, Akiyama K, Ohkoshi M, Nohara K, Tanaka T, et al. (2012) Nitrite content in various animality foods. Bull Kokusaigakuin Saitama College 33:87-94.
- Pet Food Fair Trade Council (2017) Examples of limiting additives -Sodium nitrite.
- Pharmaceutical Society of Japan ed. (2016) Methods of analysis in health science and Commentary.KaneharaShuppan Co. 359-360.
- Iijima N, Naito H, Kobayashi K, Tanabe E, Masuko T, et al. (2007) Investigation for origin of nitrite in color fixative-free meat products. Food Sanitation Res 57: 47-51.
- Tanaka A, Akiyama K, Ohkoshi M, Nohara K, Tanaka T, et al. (2012) Nitrate or nitrite content in various vegetables. Bull Kokusaigakuin Saitama College 33:17-26.
- Tanishi S, Ogawa T (2003) Food and health 2.Kagakudoujin Co. 60.
- Noda H, Minemoto M, Eto S, Noda A, Matsuyama K (1984) Nitrite ion, its stability in an aqueous solution and disappearance from rabbit blood.YakugakuZasshi 104:409-412.
- Takahashi K (1983) Use of color former for meat products. New Food Industry 25: 20-24.
- Tanimura A (1971) Reactions of food additives with foods components –In relation to nitrite-. Food Hyg Safety Sci (ShokuhinEiseigakuZasshi) 12: 277-281.
- Ohki T (1999) Pet food and food additives. J Pet AnimNutr 2: 25-30.
- Ando Y (1981) Recent problems in botulism and its control. Food Hyg Safety Sci (ShokuhinEiseigakuZasshi) 22: 455-461.
- Hioki S, Katou H, Itaya H, Shimada K, Tatsumi R, Nishimura T, Hattori A (1997) Effects of sodium nitrite and common salt on quality of sausage. Res Bull Univ Farm Hokkaido Univ 30: 55-60.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences