Morphological Characterization of Pichia guilliermondii and Saccharomyces cerevisiae Yeast and their Effects on Adherence of Intestinal Pathogens on Piglet and Chicken Epithelium In-vitro
Manfred Peisker, Elizabeth Stensrud, Juha Apajalahti, Mamduh Sifri
DOI10.21767/2572-5459.100029
Manfred Peisker1*, Elizabeth Stensrud2, Juha Apajalahti3 and Mamduh Sifri2
1ADM Specialty Ingredients (Europe), Amsterdam, The Netherlands
2ADM Specialty Ingredients, Decatur, IL, USA
3Alimetrics Ltd. Espoo, Finland
- *Corresponding Author:
- Peisker M
ADM Specialty Ingredients (Europe)
Amsterdam, The Netherlands
Tel: 49173268-8138
E-mail: manfred.peisker@adm.com
Received date: May 03, 2017; Accepted date: June 14, 2017; Published date: June 19, 2017
Citation: Peisker M, Stensrud E, Apajalahti J, Sifri M (2017) Morphological Characterization of Pichia guilliermondii and Saccharomyces cerevisiae yeast and their Effects on Adherence of Intestinal Pathogens on Piglet and Chicken Epithelium In-vitro. J Anim Res Nutr Vol No 2 Iss No 1:9 doi: 10.21767/2572-5459.100029
Copyright: © 2017 Peisker M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Intestinal pathogen binding effects are related to mannan-oligocaccharides that occur in several micro-organism species e.g. fungi and yeasts. Binding varies between species depending on their size, structure and cell wall properties. The purpose of the study was to elucidate differences in adherence potential of commercially used yeast cells and possibly determine causative elements for the observed differences. Electron microscopy and surface hydrophobicity measurements reveal that Pichia guilliermondii yeast possesses distinguished traits compared to Saccharomyces cerevisiae that may account for observed differences in pathogen binding efficiency. Pichia guilliermondii significantly inhibited adherence of pathogenic strains of Escherichia coli and Salmonella enterica in small intestinal epithelium of swine and broiler chicken. Treatment of Pichia yeast with digestive fluids significantly increased this effect. In-vivo, gastric activation occurs naturally as verified by broiler assays with P. guilliermondii. P. guilliermondii and S. cerevisiae retained their inhibitory effects on pathogenic E. coli adherence after passing through the upper digestive tract. Jejunal digesta recovered from birds treated with P. guilliermondii showed higher inhibitory effect than digesta from birds treated with the S. cerevisiae yeast. The inhibitory effect was still detectable in ileal digesta. Intact, non-gastric treated P. guilliermondii was as effective as the gastric pre-treated yeast, suggesting that digestive enzymes of broiler chicken were capable of activating yeast cells in-situ.
https://transplanthair.istanbul
https://hairclinicturkey.co
https://hairclinicistanbul.co
https://besthairtransplant.co
https://hairtransplantistanbul.co
Keywords
Yeast; Pichia guilliermondii; Mannan- Oligosaccharides; Intestinal pathogen binding
Abbreviations:
Pg: Pichia guilliermondii; Sc: Saccharomyces cerevisiae
Introduction
Despite an EU-wide ban of antibiotic growth promoters in feed, still significant levels of AGPs are used in livestock industry. Particularly worrying is the observation of the European Food Safety Authority (EFSA) and the European Centre for Disease Prevention and Control (ECDC), reporting a high resistance for commonly used antimicrobials such as ampicillin and tetracyclines. In animals and food, a very high proportion of Campylobacteria is resistant to ciprofloxacin, particularly in chicken but also in pigs and cattle. In humans, animals and food a high proportion of Salmonella and indicator Escherichia coli is resistant to common antimicrobials (EFSA 2010) [1].
Since the ban of antimicrobial growth promoters in the European Union, feed manufacturers have been actively looking for alternatives to control intestinal microbiota in production animals. The impact of mainly beta-glucans on the animals’ immune system has been largely investigated and proven by Bland et al and Soltanian et al. and will not be highlighted in this paper. The control of both food borne pathogens and pathogens causing mortality and growth suppression in production animals is especially important for economic livestock production. For the incidence of Salmonella, strict limits have been proposed by EU regulatory authorities [2,3].
Alimetrics Ltd. has developed methods to quantitatively measure the efficiency of bacterial adherence to authentic mucus recovered from target production animals. These tests, when performed in the presence or absence of potential adherents, give a measure of the power of those ingredients to prevent bacterial adherence.
This paper sheds some light on morphological and physical differences between Pichia guilliermondii and Saccharomyces cerevisiae yeast, which may be held accountable for differences between yeast cells in terms of effects in-vivo. Two yeast strains have been chosen for their commercial importance in livestock feeding. After morphological and physical assessment of the two yeast strains, studies to assess intestinal pathogen binding followed. In trial A, the efficiency of Pichia guilliermondii yeast to inhibit adherence of intestinal pathogens (E. coli F4+ and S. enterica) was assessed against pure mannose. These pathogens were chosen since they can cause serious economic damage in broiler and swine industry. In trial B, the result of trial A, i.e. that in-vitro activation of Pichia guilliermondii by gastric treatment is naturally occurring under in-vivo conditions, was verified and compared with the effect of Saccharomyces cerevisiae in a broiler trial.
Material and Methods
Cell wall glycoproteins, surface and hydrophobicity
Fluorescence microscopy was used to evaluate the distribution of yeast cell wall glycoproteins (chitin, mannose, and beta-glucans) in both yeasts. Lectins were fluorescently labeled, sugar binding proteins have a high affinity to specific sugar residues, while 1,3-β-glucan was stained with aniline blue. Concanavalin A selectively binds to mannose, specifically αmannopyranosyl and αglucopyranosyl residues, while wheat germ agglutinin (WGA) binds to N-acetylglucosamine residues found in chitin that is enriched at bud scars in the cell wall of yeast cells.
For localization studies, yeast cells were suspended in phosphate buffer saline and the larger spray-dried yeast cells were allowed to sediment to the bottom of the centrifuge tube by gravity to allow for imaging of individual and smaller agglomerated yeast cells. Both yeast products show some autofluorescence (background fluorescence without a dye) within the cells, as an artifact from fermentation and drying/cell death conditions.
For surface area, fifty individual budded Pichia guilliermondii and Saccharomyces yeast were used to calculate the size and surface area from DIC microscopy images. The surface area was calculated assuming the cells were prolate ellipsoidal.
The hydrophobicity of the yeast cell surface was evaluated by two methods - contact angle measurements and cell surface hydrophobic assay.
The contact angle of a water droplet was measured after a lawn of yeast cells was dried onto an agarose gel pad. Higher contact angle indicates a more hydrophobic yeast cell surface. Cell surface hydrophobicity (CSH) assay was also utilized to probe the hydrophobicity of the yeast cell surface by the affinity of the cells to partition into a hydrophobic solvent.
The initial absorbance (Ao) of yeast cells was measured at 600 nm; a hydrophobic solvent xylene was added to the yeast suspension, vortexed and incubated for ~ 15 minutes. Then the aqueous layer was removed and absorbance at 600 nm recorded.
CSH was calculated as CSH=(Ao-A)/Ao* 100. Larger CSH indicates that the cell surface is more hydrophobic and yeast cells partitioned from the aqueous phase into the hydrophobic solvent.
Pre-treatments of Pichia guilliermondii
Trials A & B: Spray-dried yeast was tested as-is and after gastric treatment to mimic the digestion process that ingested feed gets exposed to in the gastrointestinal tract of the host. Gastric treatment was only applied to P. guilliermondii as pilot treatment to check on possible effects. Five different doses of yeast (80, 200, 400, 800 and 2000 mg/kg) were suspended in 13.5 ml of buffer which pH was adjusted to 2.5 with 1M HCl. Then 1.5 ml of pepsin solution (1 mg/ml) was added and the cocktail incubated at 37°C for 2 hours. After the incubation, pH was elevated to 7.0 with 1M NaOH and volume was fixed to 16 ml with water. Finally, 4 ml of pancreatin solution (12.5 mg/ml) was added and incubation at 37°C continued for 2 hours. For chicken model yeast was ground with mortar and pestle before it was exposed to digestion. This mimicked the grinding function of chicken gizzard.
Preparation of mucus plates
Authentic intestinal mucus was recovered from the small intestine of two-week-old broiler chicken (trials A & B) and piglets (trial A) two weeks after weaning. The mucus samples were diluted with a buffer and cleared for all insoluble particles such as epithelial cells and bacteria by high speed centrifugation. Mucus preparations were diluted to the final protein concentration of 0.1 mg/ml. These preparations were then used to coat microtitre wells which bind mucus irreversibly. Thus a layer of mucus mimicking the surface of intestinal epithelium was introduced on the surface of the wells for the bacterial adherence studies. After overnight incubation at 4°C the wells were washed twice with Hepes Hanks buffer to wash off the unbound mucus components, and used immediately for the adherence assay.
Feeding and sampling
Broiler chicken were raised in 20 floor pens, 2 pens randomly placed per treatment with 8 birds per pen, 4 males and 4 females. Jejunal and ileal digesta was collected from 5 birds per pen, 10 birds per treatment (5 males and 5 females) and 100 birds in total. Digesta samples were frozen until used for the tests.
Preparation of labelled bacteria
Escherichia coli F4+ (A&B) and Salmonella enterica serovar Enteritidis (A) were grown in Luria broth at 37°C in a stationary culture overnight. Culturing was continued by successive transfers to fresh medium on three successive days. In the morning of the day the adherence study was to be carried out the overnight grown culture was diluted 1:10 in fresh growth medium and spiked with radioactive labelled thymidine. For two hours the culture was incubated at 37°C. During incubation bacteria were taking up the radioactive nucleotide and incorporated it in their DNA. Radioactive labelled bacteria were harvested by gentle centrifugation and re-suspended in equal volume of Hepes Hanks buffer. The suspension was used immediately for the adherence study.
Treatments
Adherence test
Trial A: Test yeast cells were introduced in mucus coated microtitre wells at volumes of 50 μl. Subsequently, radioactive labelled pathogenic bacterial suspension in Hepes Hanks buffer was added at a volume of 150 μl. The reaction mixture was incubated at 37°C for one hour. Reaction liquid was discarded and wells washed twice with 300 μl of Hepes Hanks buffer to remove unbound radioactive bacteria. Then, 250 μl of scintillation liquid was added and radioactivity measured in each reaction well. The remaining radioactivity was proportional to the number of adhered pathogenic bacteria. The number of pathogens bound in the wells without potential adherence inhibitor was compared to those with the test yeast cells. The efficiency of the yeast cells to inhibit pathogen adherence was expressed as percentage of bound pathogens.
Trial B: Digesta samples were suspended in water (1 g/100 ml), shaken for 1 hour at room temperature and centrifuged at 3000 x g for 5 min. 50 μl of supernatant were introduced directly or after 1:2 dilution in H2O in mucus coated microtitre wells. Subsequently radioactive labelled pathogenic bacterial suspension in Hepes Hanks buffer was added at a volume of 150 μl. The remainder of the procedure was as described for trial A.
Statistical analysis
Statistical analysis consisted of two-tailed t-tests for all measured parameters. The tests for each experimental diet were performed against the base diet. The pair wise tests were used to let the individual treatments be independent of the other treatments tested simultaneously. In figures statistically significant difference to base diet is indicated by asterisks as follows:
p-value<0.05*
p-value<0.01**
p-value<0.001***
Results
Morphology and physical measurements
Cell wall glycoproteins: Figure 1 shows the distribution of chitin. Both yeasts show staining of WGA, chitin, at the bud scars in the cell wall. Chitin localizes as a punctate spots in Pichia, while Saccharomyces yeast have a larger ring structures at the bud scars. The subtle staining within the entire cells is autofluorescence, as it is observed when no dye is present. Chitin localization can also be utilized to determine the age of the yeast populations, as new yeast cells will only have a single bud scar and older cells will have multiple bud scars.
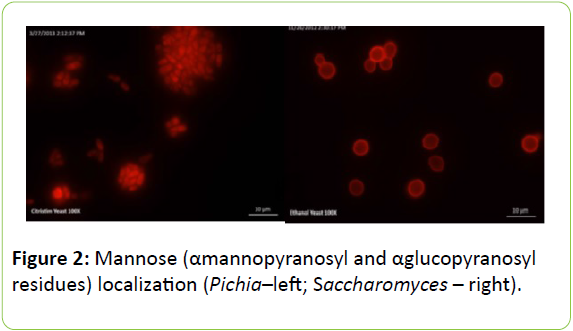
Figure 2 shows the distribution of mannose, which was determined using fluorescent microscopy with Concanavaline A. Mannose localizes around the cell periphery in Saccharomyces. Interestingly, no localization of ConA could be observed in the cell wall of Pichia above the background staining in the cell, suggesting that there could be fewer mannose residues on the cell surface and the cell wall construction of the two yeast species is different. However, a cell wall extraction and localization of other sugar residues may provide more insight into the chemical composition of the two yeast products.
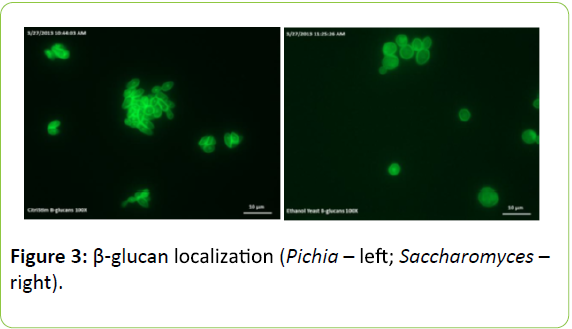
Figure 3 shows the localization of β-glucans. Fluorescence microscopy images show staining of 1,3-β-glucan around the cell periphery of Pichia and Saccharomyces yeast. Fluorescent intensity measurements of the aniline blue staining suggest that 1,3-β-glucan may be in greater concentrations around the cell periphery in Pichia but further image analysis are required to confirm this.
Surface
Table 2 shows the surface of the investigated yeast cells. Saccharomyces yeast is 2-3 times wider and longer than Pichia yeast. Saccharomyces cells have a larger surface area of 257.8 μm2 compared to 158.9 μm2 of Pichia yeast.
| Trial A (broiler & piglets) |
Mannose | (1 – 2.5 – 5 – 10 – 25 mg/ml) |
| Pichiaguilliermondii | (- “ -) | |
| Pichiaguilliermondii - Gastric treated | (- “ -) | |
| Trial B (broiler) |
Control | |
| Pichiaguilliermondii | 0.5 g/kg | |
| Pichiaguilliermondii | 1.0 g/kg | |
| Pichiaguilliermondii – Gastric treated | 0.5 g/kg | |
| Pichiaguilliermondii – Gastric treated | 1.0 g/kg | |
| Saccharomyces cerevisiae | 0.5 g/kg | |
| Saccharomyces cerevisiae | 1.0 g/kg | |
Table 1: Treatments of Trail A and Trail B with Mannose.
| Tested Yeast | Avg. Width | Avg. Length | Avg. Surface Area |
|---|---|---|---|
| P. guilliermondii | 2.68 | 4.19 | 158.9 |
| S. cerevisiae | 7.39 | 8.55 | 257.8 |
Table 2: Width (μm), Length (μm) and surface area (μm2) of budded Pichiaguilliermondii and budded Saccharomyces cerevisiae yeast (n=50)
Hydrophobicity
Table 3 shows results of hydrophobicity measurements of the assessed yeast cells. Contact angle and cell surface hydrophobicity show significantly higher hydrophobic properties for P. guilliermondii compared to S. cerevisiae yeast.
| Tested Yeast | Contact Angle (°) | CSH |
|---|---|---|
| Control (no yeast) | 59.4 | not applicable |
| P. guilliermondii | 80.1 | 21.4 |
| S. cerevisiae | 17.1 | 1.6 |
Table 3: Contact angle (°) and cell surface hydrophobicity (CSH) of Pichiaguilliermondii and Saccharomyces cerevisiae yeast
Intestinal pathogen binding trial A
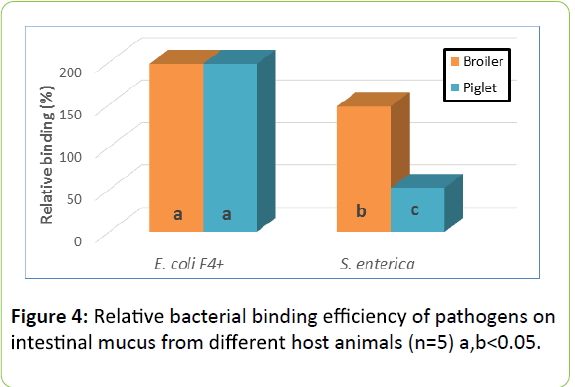
Relative binding efficiency of pathogens: Figure 4 shows the Relative efficiency of binding of two pathogen strains used in this study. E. coli F4+ adhered equally efficient to mucus recovered from small intestine of broiler chicken and piglets. The strain representing S. enterica serovar enteritidis , however, adhered more efficiently to mucus from broiler chicken.
Effect of yeast cells on pathogen adherence to chicken intestinal mucus
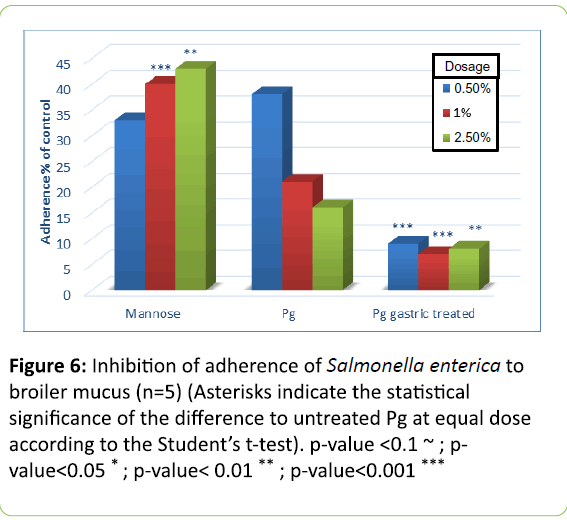
In Figures 5 to 8 adherence of pathogens in the presence of yeast cells is expressed as percentage of that shown in Figure 4, which was measured in the absence of yeast cells and set as 100% adherence. Asterisks represent the statistical Significance of the different to untreated Pichia guilliermondii (Pg) at equal dose according to Student`s t-test.
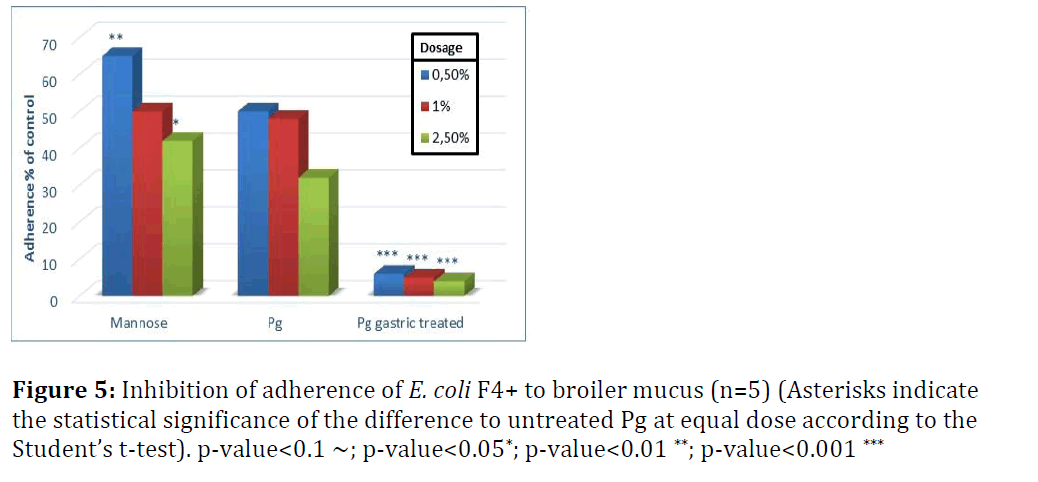
In broilers, untreated Pichia guilliermondii was more effective against S. enterica (Figure 5) than against E. coli (Figure 6). At the lowest tested dose it inhibited adherence of E. coli by 50% and that of Salmonella by more than 60%. Compared with mannose, it showed significantly better inhibition of adherence with lowest and highest dose levels for E. coli; and for S. enterica with the two highest dose levels. Untreated yeast was efficient already at the lowest tested dose and with incremental dosage infibition of adherence increased.
Gastric treatment of P. guilliermondii proved to be a strong activator of yeast cells pathogen binding effect. At every dose level the inhibition effect was significantly better compared to untreated yeast. It can be assumed that activation would occur spontaneously when yeast cells pass through the upper digestive tract, in particular the proventriculus, gizzard and duodenum. This was to be proven with trial B.
Effect of yeast cells on pathogen adherence to piglet intestinal mucus
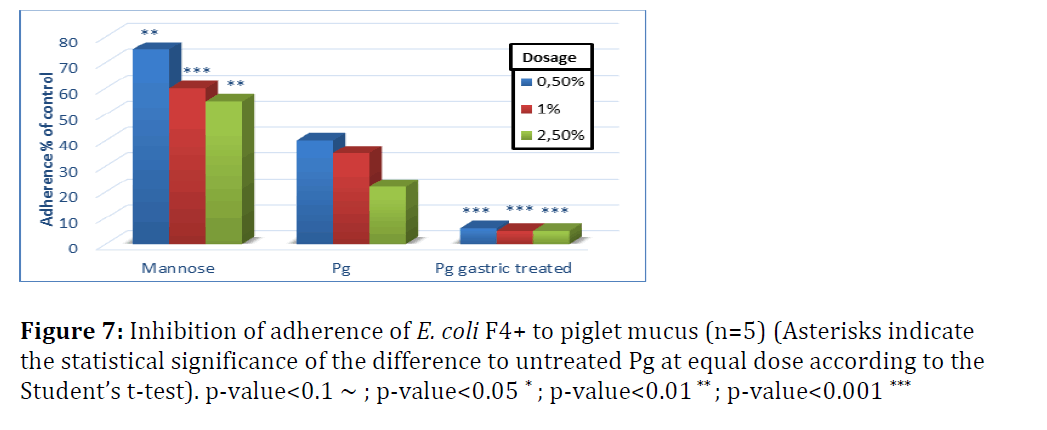
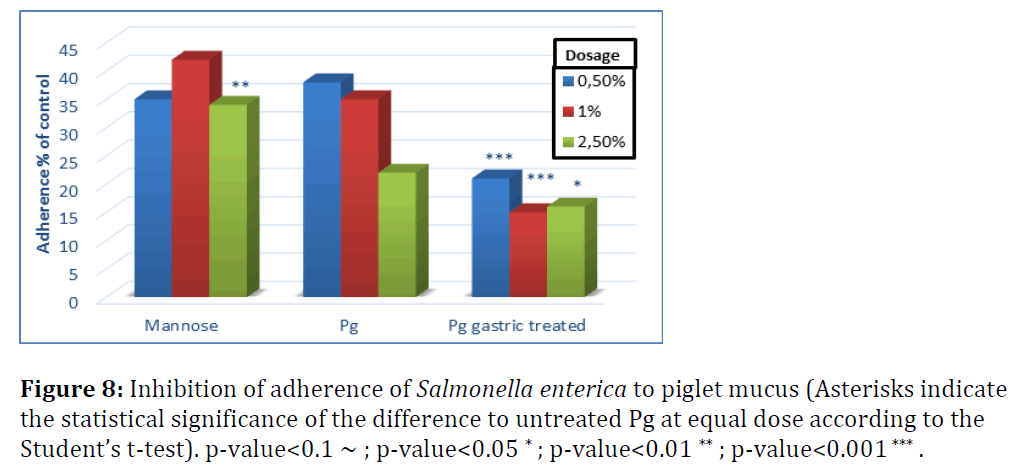
Figures 7 and 8 show the effect of yeast cells on pathogen binding with mucus from small intestine of piglets (Figure 7). Untreated yeast inhibited adherence of E. coli F4+ by 60 to 80% when the dose of yeast cells increased from 0.5 to 2.5%.
S. enterica adherence to piglet mucus was inhibited by 60–75% with untreated P. guilliermondii and not significantly different from mannose, except for the highest dose level (Figure 8). Adherence of S. enterica to piglet mucus was less efficiently inhibited compared to chicken mucus. This suggests that yeast cells tested would be more powerful inhibitors of Salmonella adherence in chicken than in piglets. Gastric treatment, as shown in broilers, significantly increased the adherence efficiency of P. guilliermondii in both species, albeit more pronounced for E. coli.
Intestinal pathogen binding trial B
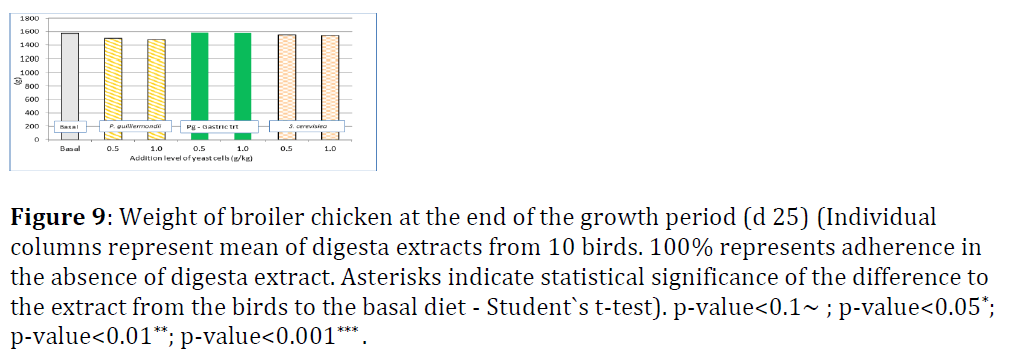
In trial B, the hypothesis was proven, that the passage of yeast cells through the intestinal tract, in particular proventriculus and gizzard, has the same effect on their binding potential as gastric pre-treatment applied in trial A. There was no significant difference in life weight of broiler chicken at day 25 (Figure 9).
Figure 9: Weight of broiler chicken at the end of the growth period (d 25) (Individual columns represent mean of digesta extracts from 10 birds. 100% represents adherence in the absence of digesta extract. Asterisks indicate statistical significance of the difference to the extract from the birds to the basal diet - Student`s t-test). p-value<0.1~ ; p-value<0.05*; p-value<0.01**; p-value<0.001*** .
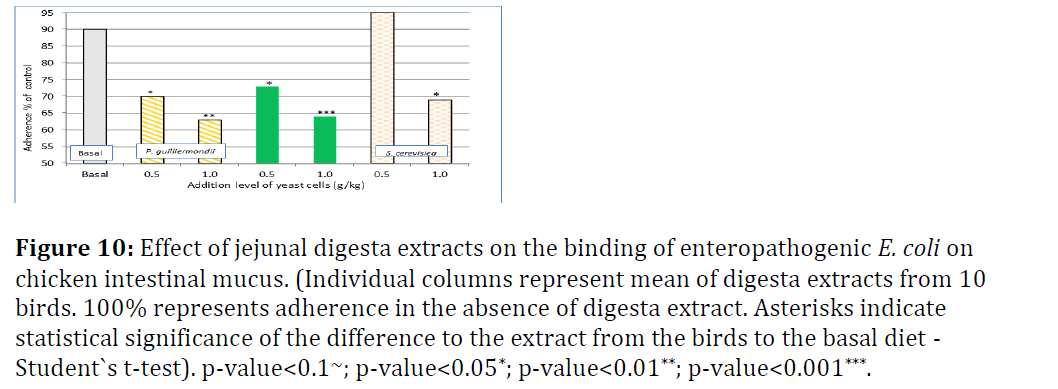
Figure 10 shows the results for adherence of E. coli to intestinal broiler mucus from jejunal digesta and Figure 8 from ileal digesta. In jejunal digesta extracts, dietary addition of Pg reduced the adherence of E. coli significantly compared to basal diet in a dose-dependent manner from 90% to 70% (0.5 g/kg) and 63% (1.0 g/kg), respectively. Gastric pre-treatment did not really alter the level of inhibition of adherence. The response with addition of 0.5 g/kg Sc did not differ significantly from basal diet. With the high Sc addition level (1 g/kg) there was significant improvement to basal diet, but none compared to 0.5 g/kg Pg addition (Figure 10).
Figure 10: Effect of jejunal digesta extracts on the binding of enteropathogenic E. coli on chicken intestinal mucus. (Individual columns represent mean of digesta extracts from 10 birds. 100% represents adherence in the absence of digesta extract. Asterisks indicate statistical significance of the difference to the extract from the birds to the basal diet - Student`s t-test). p-value<0.1~; p-value<0.05*; p-value<0.01**; p-value<0.001***.
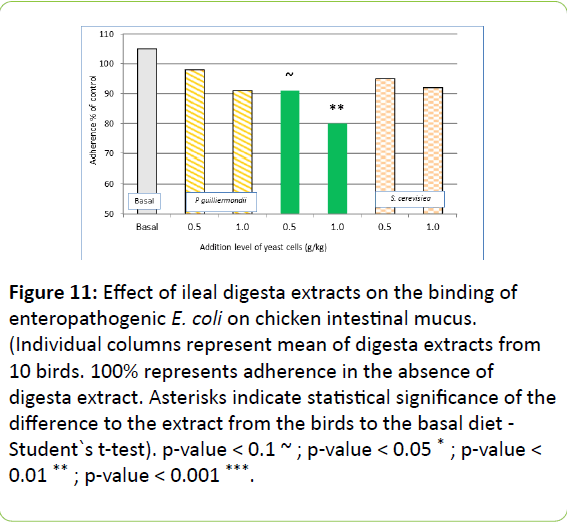
In ileal digesta extracts, the dietary addition of gastric treated Pg only reduced adherence of E. coli significantly compared to basal diet with high dose. Non pre-treated Pg and Sc did not differ in terms of inhibition of adherence of E. coli in mucus from broiler ileum (Figure 11).
Figure 11: Effect of ileal digesta extracts on the binding of enteropathogenic E. coli on chicken intestinal mucus. (Individual columns represent mean of digesta extracts from 10 birds. 100% represents adherence in the absence of digesta extract. Asterisks indicate statistical significance of the difference to the extract from the birds to the basal diet - Student`s t-test). p-value < 0.1 ~ ; p-value < 0.05 * ; p-value < 0.01 ** ; p-value < 0.001 ***.
Discussion
Many intestinal pathogens are able to colonise the gut by adhering to mucus-covered intestinal epithelium. Association with mucus is considered to be the initial phase of bacterial attachment, which may, in case of some pathogens (e.g. Salmonella) lead to the invasion of bacterial cells in epithelial cells and intestinal tissues. Salmonella is of major concern due to its pathogenicity in humans. Escherichia coli is a member of normal microbiota in many animal species and in humans. Under normal intestinal homeostasis, E. coli does not show any adverse effects on the host and its presence is linked to positive characteristics, such as vitamin K synthesis. On the other hand, some E. coli strains have harboured an ability to adhere to intestinal mucus layer that covers the epithelial cell lining and to produce a range of toxins.
The adherence of bacteria can be prevented by providing adhesin and/or receptor analogues in feed. In most cases the adherence process is complex, involves multiple mechanisms and numerous adhesin/tissue receptors, and therefore bacterial adherence often shows high degree of bacterial and host species specificity. The observation of adherence between yeast and Gram-negative bacteria (E. coli and Salmonella) is not new. It was used initially as a model to simulate and study the adherence of pathogenic bacteria to host mammalian cells (Korhonen et al.; Korhonen et al.) [4,5]. Saccharomyces boulardii has been shown to bind even cholera toxin onto its surface (Brandao et al.) [6].
The capacity to bind certain pathogenic bacteria on yeast cell surface is a well-documented characteristic of Saccharomyces yeast products. It seems that both live and heat-killed yeast cells are able to fix Salmonella typhimurium onto their surface [7-10].
Differences between yeast species appear to exist. These differences may be explained partly by morphological differences as cell size, surface structure and hydrophobicity. The degree of hydrophobicity may play an important role in bacterial adherence. Gomez-Zavaglia et al. has reported that a decrease in surface hydrophobicity lead to an increase in surface binding potential. A result of the present study does not support this statement. Gomez-Zavaglia et al. has proposed that changes in surface and adherence properties may correlate with changes in sugar components induced by bile during growth. Attribution of morphological and physical properties to binding potential of yeast cells can only be hypothesized from the present study and needs to be addressed in more detail in future [11].
As it was shown in the present study, the passage through the animals´ digestive tract seems to impart features to the yeast cells or its components, which may activate their binding potential towards pathogenic borne microorganisms (Figures 5-8). Components mediating the adherence inhibition are increased in concentration or become more efficiently exposed as adhesins or receptors when the yeast cells are disrupted or hydrolysed. However, bile salts may reduce the adherence efficiency as shown by Guglielmetti et al. and Gomez-Zavaglia et al. [11,12].
Furthermore differences seem to exist for the relative efficiency of binding for pathogens in various species. The strain of E. coli F4+ adhered equally efficient to the mucus recovered from small intestine of broiler chicken and piglets. The strain representing S. enterica serovar Enteritidis, however, adhered more efficiently to mucus from broiler chicken (Figure 7).
The effect of gastric pre-treatment was clearly visible in trial A (Figures 5-8). However, the results of trial B have shown that under in-vivo conditions there is ample activation of the yeast by the passage through the upper intestinal tract (Figure 10), which is still perceptible at ileum level (Figure 11). At equal dosage, jejunal digesta recovered from birds fed with P. guilliermondii showed higher inhibitory potential than digesta from birds fed with S. cerevisiae yeast cells. Also the inhibitory effect was still detectable in ileal digesta, suggesting that at least components or cells of yeast were retained in digesta in an effective form throughout the small intestine.
As shown in figure 9, the addition of Pg or Sc did not impact on life weight of broilers on day 25. This is similar to findings of Shanmugasundaram and Selvaraj and Shanmugasundaram et al. when broiler received Pg under non-pathogenic challenge conditions. Under challenge conditions with coccidial infection in broilers or lipopolysaccharide injection in turkeys, dietary addition of Pg mitigated the negative effect of the challenge in terms of feed intake and feed efficiency [13-15].
Conflict of Interest
Alimetrics Ltd. Espoo has no commercial association, consultancy, stock ownership, equity interest or patent-licensing agreement posing a conflict of interest with their submitted work to ADM.
References
- EFSA: wwwefsaeuropaeu/en/efsajournal/pub/2598htm
- Bland EJ, Keshavarz T, Bucke C (2004) The influence of small oligosaccharides on the immune system. Carb Res 339: 1673-1678.
- Soltanian S, Stuyven E, Cox E, Sorgeloos P, Bossier P, et al.(2009) Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit Rev Microbiol 35: 109-113.
- Korhonen TK (1979) Yeast agglutination of purified enterobacterialpili.FEMS Microbiol 6: 421-425.
- Korhonen TK, Leffler H, Svanborg EC (1981) Binding specificity of pilliated strains of Echerichia coli and Salmonella typhimurium to epithelial cells Saccharomyces cerevisiae cells and erythrocytes. Infect Immun 32: 796 -804.
- Brandao RL, Castró IM, Bambirra EA, Amaral SC, Fietto LG, et al. (1998) Intracellular signal triggered by cholera toxin in Saccharomyces boulardii and Saccharomyces cerevisiae.Appl Environ Microbiol 64: 564-568.
- Mirelman D, Altmann G, Eshdat Y (1980) Screening of bacterial isolate for mannose specific lectine activity by agglutination of yeast. J ClinMicrobiol 11: 328-331.
- Miguel JC, Rodriguez-Zas SL, Pettigrew JE (2004) Efficacy of a mannan oligosaccharide (Bio-Mos) for improving nursery pig performance. J Swine Health Prod 12: 296-307.
- Perez-Sotelo LS,Talavera-Rojas M, Monroy-Salazar HG, Lagunas-Bernabe S, Cuaron JA, et al. (2005) In-vitro evaluation of the binding capacity of Saccharomyces cerevisiae Sc47 to adhere to the wall of Salmonella. spp Rev LatinoamMicrobiol 47: 70-75.
- Tiago FCP, Martins FS, Souza ELS, Pimenta PFP, Araujo HRC, et al. (2012) Adherence to the cell yeast surface for trapping pathogenic bacteria by Saccharomyces probiotics. J Med Microbiol 61: 1194-1207.
- Gomez-Zavaglia A, KociubinskiG PP, Disalvo E, Antoni DG (2002) Effect of bile on the lipid composition and surface properties of bifidobacteria. J ApplMicrobiol 93: 794-799.
- Guglielmetti S, Tamagnini I, Minuzzo M, Arioli S, Parini C, et al. (2009) Study of the adherence of Bifidobacterium MIMBb75 to human intestinal cell lines. CurrMicrobiol 59: 167-172.
- Shanmugasundaram R, Selvaraj RK (2012) Effect of killed whole yeast cell prebiotic supplementation on broiler performance and intestinal immune cell parameters. PoultSci 91: 107–111.
- Shanmugasundaram R, Sifri M, Selvaraj RK (2013) Effect of yeast cell product supplementation on broiler cecalmicroflora species and immune responses during an experimental coccidial infection. PoultSci 92: 1195–1201.
- Shanmugasundaram R, Sifri M, Jeyabalan R, Selvaraj RK (2014) Effect of yeast cell product (Citristim) supplementation on turkey performance and intestinal immune cell parameters during an experimental lipopolysaccharide injection. PoultSci 93: 2763–2771.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences