Oral Administration of L-serine Modifies Amino Acid Metabolism in the Brain of Rats
Kazutaka Shigemi,Shozo Tomonaga,Nobuo Uotsu,Michael Denbow D,Mitsuhiro Furuse.
DOI10.21767/2572-5459.100003
Kazutaka Shigemi1, Shozo Tomonaga1, Nobuo Uotsu2, Michael Denbow, D3, Mitsuhiro Furuse1*
1Laboratory of Regulation in Metabolism and Behavior and Laboratory of Advanced Animal Bioresources, Graduate School of Bioresources and Bioenvironmental Sciences, Kyushu University, Fukuoka 812-8581, Japan
2FANCL Research Institute, 12-13 Kamishinano, Totsuka-ku, Yokohama, Kanagawa, 244-0806, Japan
3 Department of Animal and Poultry Sciences, Virginia Tech, Blacksburg, VA 24061-0306, USA
Received Date: September 06, 2015 Accepted Date: November 02, 2015 Published Date: November 05, 2015
Citation: Shigemi K, Tomonaga S, Uotsu N, et al. Oral administration of L-serine modifies amino acid metabolism in the brain of rats. J Anim Res Nutr. 2015 1:3. doi: 10.21767/2572-5459.100003
Copyright: © 2015 Shigemi K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: Whether orally administered L-serine influences amino acid metabolism in the brain was investigated. Methods: L-Serine (6 mmol/10 ml/kg) was orally administered to rats after a 9 hr fast and plasma and brain samples were obtained at 30, 60 and 120 min in Experiment 1. In Experiment 2, the effect of L-serine and glycine on brain amino acid metabolism was compared. Results: In Experiment 1, plasma serine rapidly increased 30 min after administration and then decreased, but serine in both the cerebral cortex and hippocampus gradually increased with time. Plasma taurine and glutamine were positively and leucine, phenylalanine and lysine were negatively correlated with plasma serine concentration. Cerebral cortex glutamine, citrulline, tyrosine, β-alanine, and tryptophan were negatively and α-amino adipic acid and glycine were positively correlated with serine concentration. In the hippocampus, glutamine and tyrosine were negatively correlated with L-serine. In Experiment 2, threonine concentrations were significantly decreased by L-serine treatment compared with the control (distilled water) and glycine in both brain regions. Serine concentrations in both brain regions were greatly increased by L-serine treatment, but were also moderately increased by glycine. Similarly, glycine in both regions was increased by glycine treatment and by L-serine treatment. Cysteine in both regions was increased by glycine treatment. Tyrosine in both regions was decreased by both L-serine and glycine treatments. Conclusion: Orally administered L-serine greatly influenced brain amino acid metabolism. L-Serine and glycine influenced each other, but the effect on brain amino acid metabolism was somewhat different between the two amino acids.
Keywords
L-Serine; Glycine; Cerebral cortex; Hippocampus; Plasma
Introduction
L-Serine, a nonessential amino acid, is synthesized mainly from metabolic intermediates of glycolysis, or partially from glycine. Serine-deficiency syndrome, a congenital disease associated with an inborn error generally in the L-serine biosynthetic enzyme 3-phosphoglycerate dehydrogenase, was reported [1]. Patients of this syndrome were identified because of very low concentrations of serine and glycine in the plasma and cerebrospinal fluid. Furthermore, they suffer from serious neurological symptoms such as congenital microcephaly, seizures and severe psychomotor retardation. However, these symptoms are treatable with oral supplementation of L-serine alone or in combination with glycine [1-3].
Other studies indicate that L-serine is a novel neurotrophic factor in cultured cells [4,5]. These actions are presumed to be related to metabolic products of L-serine (e.g., lipids and nucleotides) since they are components of cell membranes and nucleic acids required for neurogenesis during brain development. This idea is supported by the findings that neurons cannot maintain synthesis of serine-derived lipids without an external supply of L-serine [6,7]. These facts indicate the importance of L-serine in the central nervous system (CNS).
Asechi et al. [8] conducted a series of experiments to investigate the relationship between L-serine in the CNS and stress-related behavior of animals using the chick separation stress paradigm. Intracerebroventricular (i.c.v.) injection of L-serine and its derivatives, including glycine and L-cysteine, induced sedative and hypnotic effects under isolation-induced stress in neonatal chicks. Furthermore, it was shown that the sedative and hypnotic effects of i.c.v. L-serine were due to L-serine itself, and not its metabolites [9]. In addition, Shigemi et al. [10] exposed rats to a social isolation stress for 4 weeks, and a home cage test and open field test were included to evaluate the effect of L-serine administered orally in the water on behavior. L-Serine in the drinking water increased L-serine levels in some brain areas, but changes in its metabolites were almost negligible. L-Serine decreased locomotor activity in rats exposed to a familiar environment. In addition, L-serine decreased exploratory behavior of isolated rats, even in a novel environment. These facts suggest that daily intake of L-serine can attenuate symptoms induced by chronic stress.
L-Serine and glycine are reversibly metabolized by serine hydroxymethyltransferase. Shigemi et al. [11] investigated the involvement of glycine receptors for sedative and hypnotic effects of L-serine, since L-serine is suggested to act as the α-homomeric glycine receptor agonist [12]. Glycine similarly induced sedative and hypnotic effects in chicks, but its effect was attenuated by the glycine receptor antagonist strychnine [11]. Therefore, whether the effect of L-serine was mediated through the glycine receptor was investigated using L-serine and strychnine. The effect of L-serine was inhibited by picrotoxin, a γ-aminobutyric acid (GABA)A receptor antagonist, but not strychnine. It appears that L-serine induces sedative and hypnotic effects by enhancing inhibitory neurotransmission via GABAA receptors.
Currently, it is not known how quickly orally ingested L-serine is transported to the brain. Furthermore, the short term effect of L-serine on peripheral and central amino acid metabolism is also not known. Therefore, the present study was conducted to investigate the time course changes of peripheral and central amino acid concentrations after oral administration of L-serine. In addition, effects of L-serine on brain amino acid metabolism were compared with those of glycine.
Materials and Methods
Animals
Male Wistar rats (three weeks old) were purchased from Japan SLC, Inc. (Hamamatsu, Japan). They were kept in cages (three per cage) at a room temperature of 25 ± 1°C on a 12L:12D light-dark cycle (lights on 08:00, lights off 20:00), and given free access to a commercial diet (MF, Oriental Yeast, Co., Ltd., Tokyo, Japan) and water. They were allowed to habituate for one week before beginning the experiment. This study was performed according to the guidance for Animal Experiments in the Faculty of Agriculture and in the Graduate Course of Kyushu University and the Law (No. 105) and Notification (No. 6) of the Government.
Experimental Procedure
Following a habituation period, rats were randomly selected and divided into four groups, each with six rats. The rats were fasted for 9 hr. Except for the control group, rats were orally administered L-serine (6 mmol/10 ml/kg) in Experiment 1. The control group received no treatment. Thirty, 60, or 120 min after treatment, rats were decapitated and the blood was collected into heparinized tubes. Blood samples were centrifuged for 15 min at 4°C at 6,000 g and the plasma was removed and stored at -80°C until analysis. The cerebral cortex and hippocampus were collected, weighed, rapidly frozen with liquid nitrogen and stored at -80°C until analysis. In Experiment 2, rats were divided into three groups, i.e., the control (distilled water), L-serine (6 mmol/10 ml/kg) and glycine (6 mmol/10 ml/kg) given following a 9 hr fast. The cerebral cortex and hippocampus were collected 120 min post administration. The hippocampus was dissected according to the method of Hagihara et al. [13]. L-Serine and glycine were provided by Kyowa Hakko Bio (Tokyo, Japan).
Analysis of Free Amino acids
The method of sampling for the amino acid analysis has been described elsewhere [14] with some modifications. Brains were homogenized in ice-cold 0.2 M perchloric acid solution containing 0.01 mM EDTA·2Na and left for deproteinization on ice for 30 min. Then, the tissue homogenates were centrifuged at 20,000 × g for 15 min at 0°C. These supernatants were adjusted to pH 3 with 1 M sodium acetate and were filtrated through a 0.2 μm filter (Millipore, Bedford, USA). The plasma was diluted with sulfosalicylic acid solution (20 mg/ml) and shaken for 15 min. Then the homogenate was centrifuged at 2,000 x g for 15 min at 4ºC. Next, the sample was filtered through a 0.2-μm filter (Millipore, Bedford, USA). Then, 40 μl of each filtrate was applied to the Amino Acid Analyzer L-8800 (Hitachi High-Technologies Co. Ltd., Hitachi, Japan). Standard solutions were prepared by diluting a commercially available L-amino acid solution (type AN II and type B; Wako, Osaka, Japan) with distilled water.
Statistical Analysis
Results were subjected to one-way analysis of variance. When significant (P<0.05) effects were detected, comparisons between means were made using the Fisher PLSD test. All analyses were performed with StatView (version 5, SAS institute, Cary, N.C., United States). Results are presented as means ± S.E.M. In Experiment 1, correlations between serine and other amino acids were determined in the plasma, cerebral cortex and hippocampus.
Results
Amino acid concentrations were measured as the sum of L and D types. Thus, except for administered L-serine, amino acids were described as the name without discrimination of L and D types.
Experiment 1: Time course changes in plasma and brain amino acids after a single L-serine administration
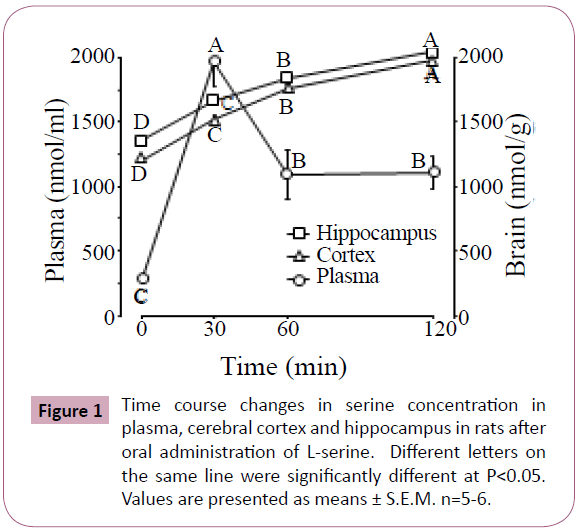
Figure 1 shows the time course of serine concentration changes in the plasma, cerebral cortex and hippocampus of rats after oral administration of L-serine. Plasma serine rapidly increased at 30 min after administration, and then decreased at 60 min although the concentrations remained higher than the initial values. Serine gradually increased with time in both the cerebral cortex and hippocampus.
Table 1 shows significant changes in plasma free amino acids after oral administration of L-serine. Among 11 amino acids, plasma taurine and glutamine were positively and leucine, phenylalanine and lysine were negatively correlated with plasma serine concentration.
| Time after administration (min) | Correlation coefficient between plasma serine | ||||
|---|---|---|---|---|---|
| 0 | 30 | 60 | 120 | ||
| Taurine | 226 ± 19 B | 290 ± 15 A | 225 ± 15 B | 237 ± 15 B | 0.503 |
| Threonine | 280 ± 8 A | 293 ± 14 A | 241 ± 17 B | 229 ± 9 B | |
| Glutamic acid | 96 ± 3 B | 123 ± 4 A | 116 ± 4 A | 116 ± 5 A | 0.708 |
| Glutamine | 897 ± 23 A | 862 ± 24 A | 753 ± 36 B | 770 ± 9 B | |
| Glycine | 368 ± 8 C | 415 ± 13 BC | 410 ± 14 BC | 479 ± 26 A | |
| Citrulline | 134 ± 5 A | 147 ± 4 A | 115 ± 9 B | 110 ± 5 B | |
| Leucine | 149 ± 6 A | 105 ± 5 B | 128 ± 20 AB | 124 ± 8 AB | -0.667 |
| Phenylalanine | 70 ± 2 A | 56 ± 1 B | 64 ± 3 AB | 65 ± 3 AC | -0.735 |
| Tyrosine | 109 ± 4 A | 100 ± 5 A | 74 ± 2B | 84 ± 4B | |
| Lysine | 578 ± 12 A | 461 ± 16 B | 439 ± 48 B | 455 ± 20 B | -0.588 |
| Hydroxyproline | 106 ± 4 A | 105 ± 3 A | 77 ± 8 B | 97 ± 7 A | |
Table 1: Significant changes in plasma free amino acids (nmol/ml) and correlation between plasma serine concentrations after oral administration of L-serine.
Table 2 shows significant changes in brain free amino acids after oral administration of L-serine. In the cerebral cortex, 9 amino acids were changed by L-serine treatment. Glutamine, citrulline, tyrosine, β-alanine and tryptophan were negatively correlated with serine concentration as shown in Figure 1. In contrast, α-amino adipic acid and glycine were positively correlated with serine. In the hippocampus, only glutamine, leucine and tyrosine were changed by L-serine treatment. Glutamine and tyrosine were negatively correlated with serine (Figure 1).
| Time after administration (min) | Correlation coefficient between | ||||
|---|---|---|---|---|---|
| 0 | 30 | 60 | 120 | ||
| Cerebral cortex | serine in the cerebral cortex | ||||
| Glutamine | 6233 ± 79 A | 6012 ± 54 A | 5669 ± 90 B | 5689 ± 102 B | -0.737 |
| a-AAA | 84 ± 5 B | 89 ± 5 B | 91 ± 4 AB | 104 ± 5 A | 0.570 |
| Glycine | 708 ± 17 B | 714 ± 14 B | 731 ± 18 AB | 777 ± 20 A | 0.541 |
| Citrulline | 66 ± 3 A | 63 ± 2 AB | 57 ± 2 BC | 59 ± 2 BC | -0.469 |
| Isoleucine | 81 ± 3 A | 71 ± 2 B | 73 ± 3 AB | 79 ± 3 A | |
| Leucine | 115 ± 3 A | 93 ± 2 C | 101 ± 1 B | 114 ± 2 A | |
| Tyrosine | 124 ± 4 A | 124 ± 3 A | 108 ± 5 B | 103 ± 5 B | -0.553 |
| b-Alanine | 47 ± 1 A | 45 ± 2 AB | 42 ± 2 BC | 41 ± 1 C | -0.615 |
| Tryptophan | 12 ± 1 A | 7 ± 2 B | 6 ± 1 B | 8 ± 1 B | -0.465 |
| Hippocampus | serine in the hippocampus | ||||
| Glutamine | 6709 ± 116 A | 6458 ± 137 A | 6027 ± 70 B | 6001 ± 105 B | -0.676 |
| Leucine | 129 ± 2 A | 104 ± 2 C | 107 ± 2 C | 117 ± 3 B | |
| Tyrosine | 135 ± 2 A | 138 ± 4 A | 117 ± 7 B | 112 ± 5 B | -0.556 |
Table 2: Significant changes in brain free amino acids (nmol/g) and correlation between brain serine concentrations after oral administration of L-serine.
Experiment 2: Changes in brain amino acids after a single L-serine or glycine administration
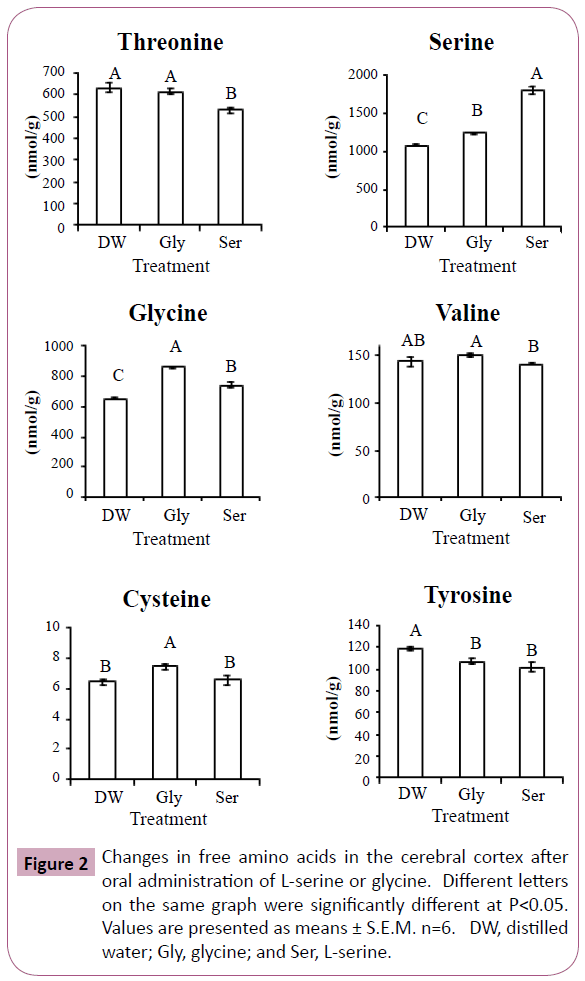
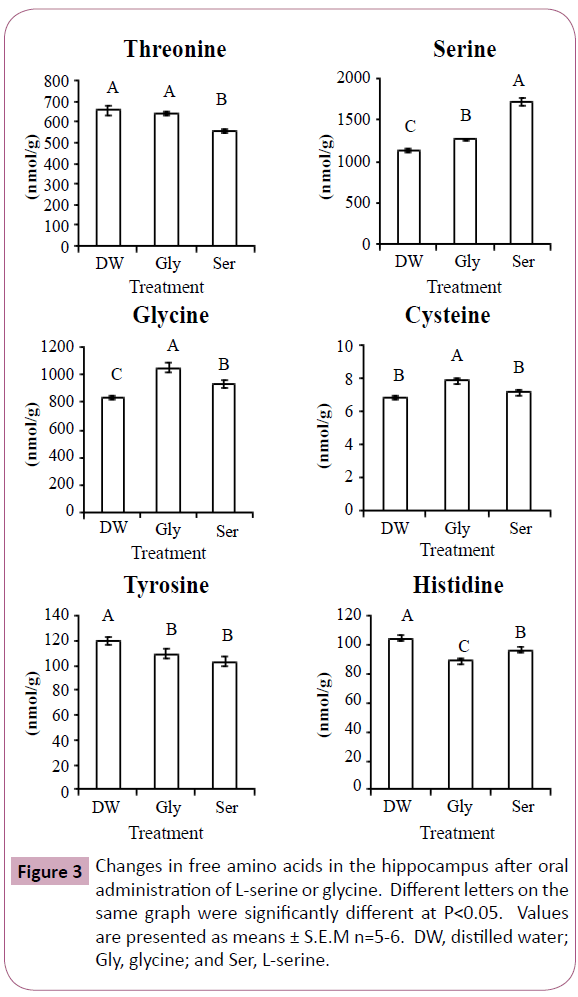
Figures 2 and 3 show significant changes in free amino acids in the cerebral cortex and hippocampus after oral administration of L-serine or glycine. Threonine, serine, glycine, cysteine and tyrosine showed similar changes in both the cerebral cortex and hippocampus. In both brain regions, threonine concentrations were significantly decreased by L-serine treatment compared with the control (distilled water) and glycine. Serine concentrations in both brain regions were greatly increased with L-serine treatment, but were also moderately increased by glycine. Similarly, glycine in both regions was increased more by glycine treatment than L-serine treatment. Cysteine in both regions was increased by glycine treatment. Tyrosine in both regions was decreased by both L-serine and glycine treatments. Valine in the cerebral cortex was highest in the glycine treatment group while lowest in the L-serine treatment. Histidine concentration was decreased in the hippocampus more by glycine than serine.
Discussion
Sedative and hypnotic effects of L-serine are induced 10 min after i.c.v. injection [9]. Asechi et al. [9] determined brain amino acid concentrations following behavioral tests and confirmed that serine itself was higher than the control, but its metabolites were not changed during the 10 min post-administration period. Following the administration of serine for 4 weeks in the drinking water, L-serine levels increased in some brain areas, but changes in its metabolites were almost negligible [10]. These results differ from the current results in which serine was acutely administered following a 9 hr fast.
In Experiment 1, we applied a single oral administration of serine following a 9 hr fast. Plasma serine concentration was sharply increased at 30 min and then decreased but maintained a still elevated level at 60 and 120 min post-administration (Figure 1). We expected that the pattern of changes in amino acid concentration would be similar between serine and glycine since they are reversibly produced by serine hydroxymethyltransferase. However, plasma glycine concentration was only elevated at 120 min after L-serine administration. As a result, no significant correlation was detected between serine and glycine. The synthesis of glycine from L-serine may not be under static equilibrium.
Taurine is a derivative of cysteine which is synthesized from serine. Plasma taurine concentration was correlated with plasma serine (Table 1). However, plasma cysteine was not significantly changed and not correlated with plasma serine (data not shown). Cysteine may be quickly utilized to synthesize taurine. The reasons that glutamic acid was positively correlated, and leucine, phenylalanine and lysine were negatively correlated with serine were unclear from the metabolic map.
In both Experiments 1 and 2, serine concentration was clearly increased in both the cerebral cortex and hippocampus by L-serine treatment. Our data support the report by Kasai et al. [15] who confirmed that L-serine crossed the blood brain barrier of rats.
In Experiment 1, more amino acid changes were seen in the cerebral cortex than in the hippocampus following L-serine administration. In both brain regions, glutamine and tyrosine were decreased with the increase of serine (Table 2). Glycine administration also decreased tyrosine concentration in the cerebral cortex and hippocampus (Figures 2 and 3). Tryptophan in the cerebral cortex also decreased with increasing serine. These results suggest that serine incorporation in the brain is a regulator of synthesis and/or release of monoamine transmitters. This was the case for nitric oxide (NO) production in which citrulline in the cerebral cortex was decreased with an increase of serine. Brain citrulline, an NO co-product, could be an index of brain NO generation via NO synthase (NOS) [16]. L-Serine is converted to the enantiomer D-serine by serine racemase, which was purified from the mammalian brain [17,18]. D-Serine inhibits neuronal NOS but not endothelial NOS [19]. Orally ingested serine may contribute to lower NOS activity.
In Experiment 1, glycine concentration was increased in the plasma and cerebral cortex by L-serine administration, but not in the hippocampus. This was due to serine hydroxymethyltransferase which reversibly metabolizes L-serine and glycine. Similarly, serine concentration was increased by glycine treatment in Experiment 2 (Figures 2 and 3).
Cysteine is a metabolite of serine, but cysteine concentration in the cerebral cortex and hippocampus was not increased after L-serine treatment. Glycine rather than L-serine was effective in increasing cysteine concentration. In the cysteine synthesis pathway, glycine is present up-stream of L-serine. Large amounts of L-serine may down regulate the enzymes of cysteine synthesis. In contrast, the amount of serine produced from glycine may match the enzyme activity.
Threonine and serine are metabolized by the same enzyme, serine (threonine) dehydrate. The reduction in threonine of the cerebral cortex and hippocampus by L-serine treatment may be associated with serine dehydrate. However, the precise mechanism is uncle ar.
Shigemi et al. [10] reported the mechanism by which i.c.v. L-serine induced sedative and hypnotic effects in neonatal chicks exposed to acute stressful conditions. The involvement of GABAA receptors on the effect of L-serine was investigated using the GABAA receptor antagonist picrotoxin. Co-administration of picrotoxin attenuated the sedative and hypnotic effect of L-serine. Further, Shigemi et al. [10] also investigated the involvement of glycine receptors since L-serine is suggested to act as the α-homomeric glycine receptor agonist [12]. Glycine similarly induced sedative and hypnotic effects in chicks, but its effect was attenuated by the glycine receptor antagonist strychnine. However, the effect of L-serine was not inhibited by strychnine. In the present study, L-serine did not change GABA concentrations in either Experiment 1 or 2. Accordingly, we believe that serine stimulates GABA release but not GABA synthesis. For instance, L-pipecolic acid (L-PA) is a major metabolic intermediate of L-lysine in the mammalian [20,21] and chick brain[22]. L-PA is incorporated into the crude synaptosomal fraction[23] and stimulates some brain sites in the rat[24]. It has also been reported that L-PA was able to increase the release of GABA stimulated by mild depolarization with potassium from brain slices and decrease the uptake of GABA by brain[25]. L-PA has similar effects such as hypnosis and sedation[26] as ob served by L-serine [8, 9,11].
In conclusion, orally administered L-serine greatly influenced both plasma and brain amino acid metabolism. These changes may be related to the central functions of L-serine. L-Serine and glycine influence the metabolism of each other, but the effect on brain amino acid metabolism was somewhat different between the tw o amino acids.
Conflict of Interest
Declared none.
Acknowledgement
This work was supported by a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (No. 23248046).
References
- Jaeken J, Detheux M, Van Maldergem L, Foulon, M, Carchon H, et al. (1996) 3-Phosphoglycerate dehydrogenase deficiency: an inborn error of serine biosynthesis. Archives of Disease in Childhood 74: 542-545.
- de Koning T.J, Duran M, Van Maldergem L, Pineda M, Dorland L, et al. (2002) Congenital microcephaly and seizures due to 3-phosphoglycerate dehydrogenase deficiency: outcome of treatment with amino acids. Journal of Inherited Metabolic Disease 25: 119-125.
- de Koning T.J, Klomp L.W, van Oppen A.C, Beemer F.A, Dorland L, et al. (2004) Prenatal and early postnatal treatment in 3-phosphoglycerate-dehydrogenase deficiency. Lancet 364: 2221-2222.
- Mitoma J, Furuya S, Hirabayashi Y. (1998) A novel metabolic communication between neurons and astrocytes: non-essential amino acid L-serine released from astrocytes is essential for developing hippocampal neurons. Neuroscience Research 30: 195-199.
- Furuya S, Tabata T, Mitoma J, Yamada K, Yamasaki M, et al. (2000) L-Serine and glycine serve as major astroglia-derived trophic factors for cerebellar Purkinje neurons. Proceedings of the National Academy of Sciences of the United States of America 97: 11528-11533.
- Mitoma J, Kasama T, Furuya S, Hirabayashi Y (1998) Occurrence of an unusual phospholipid, phosphatidyl-L-threonine, in cultured hippocampal neurons. Journal of Biological Chemistry 273: 19363-19366.
- Yoshida K, Furuya S, Osuka S, Mitoma J, Shinoda Y, et al. (2004) Targeted disruption of the mouse 3-phosphoglycerate dehydrogenase gene causes severe neurodevelopmental defects and results in embryonic lethality. Journal of Biological Chemistry 279: 3573-3577.
- Asechi M, Tomonaga S, Tachibana T, Han L, Hayamizu K, et al. (2006) Intracerebroventricular injection of L-serine analogs and derivatives induces sedative and hypnotic effects under an acute stressful condition in neonatal chicks. Behavioural Brain Research 170: 71-77.
- Asechi M, Kurauchi I, Tomonaga S, Yamane H, Suenaga R, et al. (2008) Relationships between the sedative and hypnotic effects of intracerebroventricular administration of L-serine and its metabolites, pyruvate and the derivative amino acids contents in the neonatal chicks under acute stressful conditions. Amino Acids 34: 55-60.
- Shigemi K, Tsuneyoshi Y, Yamada S, Kabuki Y, Hayamizu K, et al. (2010) Oral administration of L-serine reduces the locomotor activity of socially isolated rats. Neuroscience Letters 468: 75-79.
- Shigemi K, Tsuneyoshi Y, Hamasu K, Han L, Hayamizu K, et al. (2008) L-Serine induces sedative and hypnotic effects acting at GABA(A) receptors in neonatal chicks. European Journal of Pharmacology 599: 86-90.
- Schmieden V, Betz H (1995) Pharmacology of the inhibitory glycine receptor: agonist and antagonist actions of amino acids and piperidine carboxylic acid compounds. Molecular Pharmacology 48: 919-927.
- Hagihara H, Toyama K, Yamasaki N, Miyakawa T (2009) Dissection of hippocampal dentate gyrus from adult mouse. Journal of Visualized Experiments 33: 1543.
- Tomonaga S, Kaji Y, Tachibana T, Denbow D.M, Furuse M, et al. (2005) Oral administration of β-alanine modifies carnosine concentrations in the muscles and brains of chickens. Animal Science Journal 76: 249-254.
- Kasai Y, Tachikawa M, Hirose S, Akanuma S, Hosoya K, et al. (2011) Transport systems of serine at the brain barriers and in brain parenchymal cells. Journal of Neurochemistry 118: 304-313.
- Igarashi K, Sugiyama Y, Kasuya F, Inoue H, Matoba R, et al. (2000) Analysis of citrulline in rat brain tissue after perfusion with haloperidol by liquid chromatography-mass spectrometry. Journal of Chromatography. B, Biomedical Sciences and Applications 746: 33-40.
- Konno R (2003) Rat cerebral serine racemase: amino acid deletion and truncation at carboxy terminus. Neuroscience Letters 349: 111-114.
- Wolosker H, Blackshaw S, Snyder S.H (1999) Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proceedings of the National Academy of Sciences of the United States of America 96: 13409-13414.
- Darra E, Ebner F.H, Shoji K, Suzuki H, Mariotto S, et al. (2009) Dual cross-talk between nitric oxide and D-serine in astrocytes and neurons in the brain. Central Nervous System Agents in Medicinal Chemistry 9: 289-294.
- Giacobini E, Nomura Y, Schmidt-Glenewinkel T (1980) Pipecolic acid: Origin, biosynthesis and metabolism in the brain. Cellular and Molecular Biology 26: 135-146.
- Schmidt-Glenewinkel T, Nomura Y, Giacobini E (1977) The conversion of lysine into piperidine, cadaverine and pipecolic acid in the brain and other organs of the mouse. Neurochemical Research 2: 619-637.
- Nomura Y, Schmidt-Glenewinkel T, Giacobini E (1978) In vitro formation of piperidine, cadaverine and pipecolic acid in chick and mouse brain during development. Developmental Neuroscience 1: 239-249.
- Meek JL (1974) Uptake and metabolism of piperidine and pipecolic acid in brain. Federation Proceedings 1453: 468.
- Kase Y, Takahama K, Hashimoto T, Kaisaku J, Okano Y, et al. (1980) Electrophoretic study of pipecolic acid, a biogenic imino acid, in the mammalian. Brain Research 193: 608-618.
- Gutierrez MC, Delgado-Coello BA (1989) Influence of pipecolic acid on the release and uptake of [3H] GABA from brain slices of mouse cerebral cortex. Neurochemical Research 14: 405-408.
- Takagi, T, Bungo T, Tachibana T, Saito ES, Saito S, et al. (2003) Intracerebroventricular administration of GABA-A and GABA-B receptor antagonists attenuate feeding and sleeping-like behavior induced by L-pipecolic acid in neonatal chicks. Journal of Neuroscience Research 73: 270-275.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences