The Effect of Dietary Wheat Gluten Products on Gut Morphology, Non-Specific Immune Parameters, and Allochthonous Intestinal Microbial Population of Juvenile Rainbow Trout (Oncorhynchus mykiss) Fed with a High Plant Protein Diet
Voller SW, Merrifield DL, Apper E
DOI10.21767/2572-5459.100042
Voller SW1, Merrifield DL1 and Apper E2*
1Aquaculture and Fish Nutrition Research Group, School of Biological Sciences, CARS, Plymouth University, UK
2Research and Innovation Department, Tereos, ZI et Portuaire, 67390 Marckolsheim, France
- *Corresponding Author:
- Apper E
Research and Innovation Department Tereos
Z.I. et Portuaire 67390 Marckolsheim
France
Tel: 0033388581611
E-mail: emmanuelle.apper@tereos.com
Received date: January 23, 2018; Accepted date: February 16, 2018; Published date: February 23, 2018
Citation: Voller SW, Merrifield DL, Apper E (2018) The Effect of Dietary Wheat Gluten Products on Gut Morphology, Non-specific Immune Parameters, and Allochthonous Intestinal Microbial Population of Juvenile Rainbow Trout (Oncorhynchus Mykiss) Fed with a High Plant Protein Diet. J Anim Res Nutr Vol No 3: Iss no: 1: 4.
Copyright: © 2018 Voller SW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Diets for Salmonids contain less fishmeal and more plant (and especially pulse) proteins that can impact gut microbiota, morphology, and non-specific immune system. The objective of the study was to investigate if including 10% of three different types of wheat proteins in a soy-based diet affects in the same manner gut microbiota, morphology and non-specific immune parameters of juvenile rainbow trout.
Methods: Over the course of a 66 day nutrition feed trial, triplicate tanks of juvenile rainbow trout (31 fish per tank; initial weight=24.80 ± 0.31 g) were fed three experimental diets containing 10% inclusions of varying types of wheat proteins. Wheat protein inclusions, vital (VWG), hydrolysed (HWG) and a soluble hydrolysed (SWG) were incorporated into a basal feed at the expense of soy protein concentrate (SPC diet). Growth performance was monitored throughout the trial. At the end point intestinal samples were taken for microbial, molecular and histological analysis.
Findings: Growth performance was unaffected by 10% dietary inclusions of wheat proteins, especially because growth already achieved high level with the SPC diet, with a feed conversion ratio of 0.99. Modulation of the allochthonous intestinal microbiota at genus level was observed, with increased proportions of Weissella in the 10% VWG treatment compared to the SPC and SWG products. Bacillus relative abundance was significant increased with 10% SWG diet. Leuconostoc was significantly higher with HWG diet. Transcription level expression of TNF-α and Heat Shock Protein 70 (HSP 70) was significantly down-regulated in all wheat protein diets compared to the SPC treatment. A PCA performed on these parameters revealed that SPC diet was significantly associated with the first dimension characterised by high relative abundance of TNF-α, IL10, TGF-β, Glute ST, IL8 and HSP70; while SWG diet was negatively associated with the second dimension which was positively correlated with high relative abundance of IL8 and HSP70 and negatively correlated with relative abundance of IL10 and TGF-β. Increased Intra-epithelial leukocytes (IEL) counts and lamina propria width was observed with 10% VWG and HWG inclusion.
Conclusions: All types of wheat proteins are promising plant protein source with the benefits of enhancing growth of potential beneficial bacteria in the intestine, reducing intestinal stress and potentially enhancing the non-specific immune system of rainbow trout fed with a low fishmeal, high pulse diet.
Keywords
Wheat proteins; Fish; Gut; Microbiota; Intestinal gene expression
Abbreviations
VWG: Vital Wheat Gluten; HWG: Hydrolysed Wheat Gluten; SWG: Soluble Wheat Gluten; HSP70: Heat Shock Protein 70; IEL: Intra-epithelial leukocytes.
Introduction
As the ever increasing drive for sustainability in the aquaculture industry continues, there is now greater scrutiny on alternative protein sources that have the potential to raise the standard of the industry by reducing fishmeal. As the global production volume of fishmeal has plateaued at around 6 million tonnes, and with ever increasing cost, research efforts into alternative protein sources for inclusion into aqua feeds has greatly increased over the past two decades [1-5]. Advances in aqua feed formulations have been driven in part by the aqua feed producers, striving for maximum benefits from a least cost formulation strategies. Consequently feed ingredient producers are now actively seeking economically viable and suitable protein sources to meet public perception for ethical animal production [6].
Wheat proteins, namely vital and hydrolyzed wheat gluten, high protein products from the removal of starch from cereal grains are promising proteins, receiving some attention as a partial replacer for fishmeal [7-9]. Such studies have revealed no detrimental effects of the protein source on growth performance with inclusion levels up to 30% in fishmeal-based diets. Throughout the literature there is a paucity of information on the effect of wheat proteins on gut health and the intestinal microbiota, which are recognized to contribute to mucosal barrier function, producing antimicrobial substances as well as providing physical site competition and protecting the host from potential pathogens [10]. Wheat proteins are rich in glutamine which is a major energy source for rapidly proliferating cells, and involved in many other vital functions, like oxidative stress or gluconeogenesis. Thus, it can be hypothesized that addition of wheat proteins may be beneficial for gut. No comparison between the different types of wheat proteins on gut morphology, non-specific immune parameters, and microbiota has been undertaken, while the availability of amino-acids and especially of glutamine may be different. The objectives of this study were thus to assess the impacts of three varying types of wheat proteins, vital wheat gluten and two types of hydrolyzed wheat gluten on the growth performance and intestinal health of juvenile rainbow trout fed low fishmeal and high soy protein concentrate diets. We hypothesized that wheat proteins might improve gut microbiota and non-specific immune parameters compared to the control diet and that these effects might be greater with hydrolyzed wheat proteins.
Materials and Methods
Trial has been performed in agreement with the guidelines of the Directive 2010/63/EU.
Experimental diets
Four experimental diets low in fishmeal content were formulated insuring all minimum nutritional requirements were met for rainbow trout (Table 1). Three wheat protein products, vital wheat gluten (VWG, Amytex®, Tereos Starch and Sweeteners Europe, France) and 2 different hydrolyzed wheat gluten (HWG, Solpro508®, and SWG, Meripro810®; Tereos Starch and Sweeteners Europe, France) were included at 10% to a basal formulation (SPC) at the expense of soy protein concentrate (Table 1). According to the specifications of the producer, the peptide size distribution in the HWG (% of SDS buffer soluble protein) was: F1 N 779.6 kDa, 0.8%; 779.6 kDa N F2 N 96.7 kDa, 3.8%; 96.7 kDa N F3 N 58.1 kDa, 2.2%; 58.1 kDa N F4 N 20.5 kDa, 19.7%; 20.5 kDa N F5 N 6.2 kDa, 62.3%; 6.2 kDa N F6 N 0.47 kDa, 11.2%. SWG was obtained from HWG, after removal of the last insoluble protein fraction. Diets were produced and cold pressed at the University of Plymouth, Devon, UK.
| Diets | ||||
| SPC | 10% VWG | 10% HWG | 10% SWG | |
| Ingredient (g /Kg) | ||||
| Herring meal7 | 10 | 10 | 10 | 10 |
| Soya protein concentrate1 | 52 | 39.62 | 38.77 | 38.49 |
| Soyabean meal8 | 10 | 10 | 10 | 10 |
| Hydrolysed wheat gluten3 | - | - | 10 | - |
| Vital wheat gluten4 | - | 10 | - | - |
| Soluble wheat gluten5 | - | - | - | 10 |
| Corn starch6 | 7.63 | 10.65 | 10.89 | 11.33 |
| Fish oil2 | 16.49 | 15.98 | 16.55 | 16.46 |
| L-Lysine HCl6 | 1.85 | 1.72 | 1.76 | 1.69 |
| Calcium carbonate9 | 1 | 1 | 1 | 1 |
| Vitamin mineral premix10 | 0.5 | 0.5 | 0.5 | 0.5 |
| CMC-Binder6 | 0.5 | 0.5 | 0.5 | 0.5 |
| Antioxidant mix11 | 0.03 | 0.03 | 0.03 | 0.03 |
| Proximate composition (%) | ||||
| Moisture | 4.36 | 4.33 | 4.14 | 3.96 |
| Protein | 48.04 | 48.51 | 48.41 | 49.08 |
| Lipid | 19.56 | 19.47 | 19.51 | 19.55 |
| Ash | 6.11 | 5.45 | 4.49 | 4.38 |
| Energy (MJ/Kg) | 22.55 | 22.06 | 22.7 | 22.81 |
| 1SPC60 (BioMar, DK); 2Epanoil (Seven Seas, UK); 3Solpro508® (Tereos, FR); 4Amytex®(Tereos, FR); 5Meripro810®(Tereos, FR); 7LT94 Herring meal (CC Moore, UK); 6(sigma Aldrich, UK); 8HP 100 (Hamlet, DK); 9(Fisher Scientific, USA); 10PNP Fish: Ash 78.7%, Ca 12.1%, Mg 1.56%, P 0.52%, Cu 0.25 g/kg, Vit. A 1.0 μg/kg, Vit D3 0.1 μg/kg, Vit. E 7 g/kg (Premier Nutrition, UK); 11Ethoxyquin 0.075 gKg-1, BHT 0.05 gKg-1, Natural tocopherols 0.2 gKg-1 (Premier Pet Nutrition, UK). | ||||
Table 1: Dietary formulation and proximate composition (%).
Experimental design
Four hundred and sixty five rainbow trout were graded and randomly distribute into 12, 120 L fibreglass tanks (31 fish per tank; initial average body weight=24.80 ± 0.31 g) in a 7,000 litre closed recirculation system at the Aquatic Animal Nutrition and Health Research Facility at the University of Plymouth. Dietary treatments were randomly attributed to triplicate tanks and fed at a rate of 1.5-2.5% of biomass per day in equal rations at 09:00, 13:00 and 17:00 over the course of a 66 day nutritional feed trial. Feed was adjusted daily on a predicted FCR of 1.2, based on initial biomass weights, and subsequent bi-weekly tank biomass weighing data. Rainbow trout were maintained at 15 ± 1°C with a 12:12 L:D photoperiod. pH was maintained at 7.0 ± 0.5 and dissolved oxygen >85% saturation.
Growth performance
Throughout the course of the feeding trial, tank biomass was in bulk weighted bi-weekly, and prior to end point sampling to allow the calculation of growth by fish weight, feed conversion ratio (FCR), protein efficiency ratio (PER), specific growth rate (SGR), K factor, and survival. FCR was calculated as FCR=g dietary intake × (g weight gain)−1. PER was calculated as PER=g dietary crude protein intake × (g weight gain)−1. SGR was calculated as SGR (%body weight gain/day)=[(Logn Final fish weight-Logn Initial fish weight)/Time Interval] × 100. K factor was calculated by the following formula: K=10NW/L3; wherein N=5; W is the weight of the fish in grams (g), L is the length of the fish in millimetres (mm), measured from the tip of the snout to the rear edge of the fork at the centre of the tail fin.
DNA extraction and PCR
At the conclusion of the 66 day feed trial, two fish per tank (n=6 per treatment) were euthanised by overdose (200 mg l-1 water for 10 min) of tricaine methane sulphonate (MS222; Pharmaq, Fordingbridge, UK) followed by destruction of the brain. Digesta samples were removed under aseptic conditions for posterior allochthonous intestinal microbiota analysis as described by Merrifield et al. [11] and stored at -20°C. DNA extraction occurred on 100 mg digesta samples using the PowerFecal™ DNA isolation kit (Cambio, Cambridge, UK) with the addition of a lysis step prior to the manufacturers protocol.
PCR amplification was achieved utilising the reverse 338R (5’- GCW GCC WCC CGT AGG WGT-3’) and forward 27F (5’-AGA GTT TGA TCM TGG CTC AG-3’) primers, diluted to 50 pmol μl-1 (Eurofins MWG, Ebersberg, Germany). 30 μl reactions were carried out utilising the following reagents: 15 μl MyTaq™ (Bioline, London, UK), 1 μl 338R and 1 μl 27F primer, 9 μl molecular grade water and 4 μl DNA templates. The PCR conditions comprised an initial denaturing period of 7 minutes at 94°C, followed by 10 touchdown cycles of 30 sec at 94°C, 30 sec at 62°C (reducing by 1°C per cycle) and 30 sec at 72°C. This was then followed by a further 25 cycles 94°C for 30 sec, 53°C for 30 sec, 72°C for 30 sec and a final extension of 72°C for 7 minutes.
PCR products were purified using Agencourt AMPure XP (Beckman Coulter, Ca, USA) and quantified with a Qubit® 2.0 Fluorometer (Invitrogen, Ca, USA). Amplicons fragment concentrations were then assessed using an Ion Library Quantitation Kit (Life Technologies™, USA) and then adjusted to 26 pM. Amplicons were attached to Ion Sphere Particles using Ion PGM Template OT2 200 kits (Life Technologies™, USA) according to the manufacturer’s standard protocol. Multiplex sequencing was carried out with Ion Xpress Barcode Adapters (1-16 Kit; Life Technologies™) on a 316™ chip (Life Technologies™) on an Ion Torrent Personal Genome Machine (Life Technologies™). Sequences were binned by sample and filtered to remove low quality reads within the PGM software. Data were exported as FastQ files.
Taxonomic analysis of sequence reads was conducted with FASTX-Toolkit (Hannon Lab, USA) after the removal of low quality scores (Q score<20). De-noising and analysis of sequences were conducted with QIIME. OTU mapping was performed utilising the default pipeline of QIIME with USEARH removing chimeras (putative erroneous reads). Greengenes database was used for the assignment of taxonomic classification of OTUs utilising the RDP classifier, which clustered the sequences at 97% similarity with a 0.80 confidence threshold. Multiple alignments of the representative sequences for each OTU were created using PyNAST with a minimum sequence length of 150 base pairs (bp) and 97% identification. Utilising the 16S microbial Nucleotide BLAST-NCBI database, highest homologous species or genera were identified (>98% similarity at 150 bp).
Gene expression
RNA extraction and cDNA synthesis
Two fish per tank (n=6 per treatment) were sampled for gene expression analysis. Total RNA extraction from 100 mg posterior intestine was conducted using TRIzol (Invitrogen, Carlsbad, CA, USA). RNA purity and concentration was assessed using a NanoDrop™ spectrophotometer (NanoDrop Technologies, Wilmigton, USA) and stored at -20°C prior to use. Total RNA was treated with TURBO DNA-free™ (Thermon Fisher Scientific, Ma, USA). cDNA synthesis was carried out utilising iScript cDNA Synthesis Kit (Bio-Rad CA, USA), with 1 mg RNA template in a 20 μl reaction. cDNA was stored at -20°C until analysis.
Quantitative real time PCR
PCRs were performed in an iQ5 iCycler thermal cycler (Bio- Rad) following the SYBR green methodologies. qPCR reactions were carried utilising 7.5 μl reactions. Reagents utilized in triplicate reactions per dilution were as follows: 2 μl of diluted (1:10) cDNA, 3.75 μl 2X concentrated iQ™ SYBR Green Supermix (Bio-Rad), (SYBR Green was the fluorescent intercalating agent), 0.225 μl of forward and reverse primers (0.45 μl total at 0.3 μM concentration) and 1.3 μl of DEPC treated H20 (Thermo fisher scientific). Thermal cycling conditions were as follows: 10 min at 95°C, 40 cycles of 15 sec at 95°C, 60 sec at 60 °C (58°C for primers with 58°C annealing temperatures) with fluorescence recorded at the end of each cycle. Reactions and quality control measures were carried out in accordance with the MIQE guidelines. Additional melt curve (dissociation curve) analysis was carried out to ensure single peaks in all cases. β actin and elongation factor 1α were utilized as housekeeping genes. Primers for genes were designed utilizing Primer3web v.4.0.0 (www.Primer3.ut.ee) and ordered from Eurofins MWG Operon’s oligo synthesis service (Ebersberg, Germany). Specific genes of interest and primers utilised can be found in Table 2.
| Gene | Forward primer sequence (5’-3’) | Reverse primer sequence (5’-3’) | Amplicon size (bp) | Acc. No | E-value | Annealing temp |
|---|---|---|---|---|---|---|
| β actin | ACTGGGACGACATGGAGAAG | CCACCCTCAGCTCGTTGTAG | 57 | AJ438158.1 | 2 | 60 |
| EF1-α | AGGCTCCATCTTGGCTTCTC | GGGACCAGACTCGTCGTACT | 76 | AF498320.1 | 2.1 | 60 |
| IL-8 | CGGAGAGCAGACGTATTGGTAA | GAGCTGGGAGGGAACATCTC | 58 | HG917307.1 | 1.9 | 60 |
| TNF- α | TGGGTGTGAGTGACATCGTTAT | AGACCCTCAGCATCTGGTACT | 87 | HE717002.1 | 2.1 | 60 |
| Glute-ST | CCTTCTCATTGGCTGACGTTAT | GCCGTAGACAGCCCAAAG | 70 | NM_001160559.1 | 2 | 58 |
| IL-10 | GCTGGACGAAGGGATTCTACA | GCACCGTGTCGAGATAGAACTT | 89 | NM_001245099.1 | 2.1 | 60 |
| TGF-β | TGCCTTGTGATTGTGGGAAAC | CCTCAGCTTGTTCATCCCTGAT | 68 | AJ007836.1 | 2.1 | 60 |
| HSP70 | TTGGCCGCAGGTTTGATGAT | CTTCAAAGGGCCAATGCTTCAT | 60 | K02550.1 | 2.1 | 60 |
Table 2: Primer information used for real-time PCR analysis.
Data were analyzed utilizing the iQ5 optical system software version 2.0 (Bio- Rad) containing Genex Macro iQ5 Conversion and genex Macro iQ5 files. The spreadsheet calculations are based on the algorithms of Vandesompele et al. [12] and the GeNorm manual (https://medgen.ugent.be/~jvdesomp/genorm/). Delta CT levels were normalized with a normalization factor (NF), generated from the two housekeeping genes in GeNorm, to produce a normalized expression level (NEL) per gene. The formulae utilized were as follows: ΔCT=primer efficiency^ (CT value-minimum CT value observed in gene or interest). NEL=ΔCT/NF. Descriptive statistics and NELs per treatment per gene were then analyzed utilizing RStudio (available at https: //www.rstudio.com/products/rstudio/download2/), and pairwise comparisons were carried out utilizing permutation tests. Significance was accepted as P<0.05.
Scanning electron microscopy
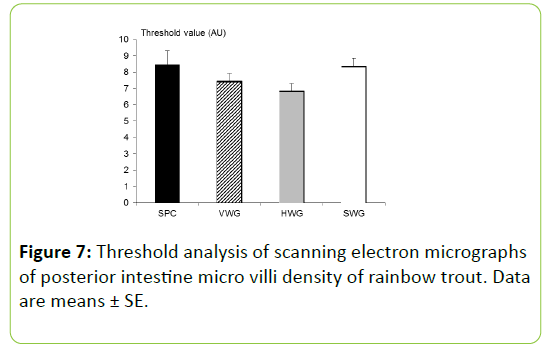
The posterior intestines of two fish per tank (n=6 per treatment) were sampled for scanning electron microscopy. After washing in 1% S-methyl-L-cysteine, phosphate buffered saline and fixation in 2.5% glutaraldehyde in pH 7.2, 0.1 M sodium cacodylate buffer samples were removed from the fixative and rinsed twice in 0.1 M sodium cacodylate buffer by immersion for 15 minutes, twice. Post washing, samples were alcohol dehydrated through graded ethanol and then critical point dried (Emitech K850, Kent, UK) utilizing ethanol as the intermediate fluid and CO2 as the transitional fluid. Dried samples were gold sputter coated (Emitech K550, Kent, UK) after being mounted on aluminium stubs using fine silver paint (Ag in methyl isobutylkelone). Electron micrographs were taken with a JSM 6610 LV (Jeol, Tokyo, Japan) SEM, and analyzed for qualitative gross structure damage and tissue necrosis.
Microvilli density (arbitrary unitsÃÆÃÂÃâþ AU) was calculated utilising ImageJ 1.45. Electron micrographs of microvilli taken at X20, 000 magnifications were threshold adjusted to a consistent point, and a ratio of background (gaps between microvilli) and foreground (microvilli) was calculated, achieving a density unit.
Histology
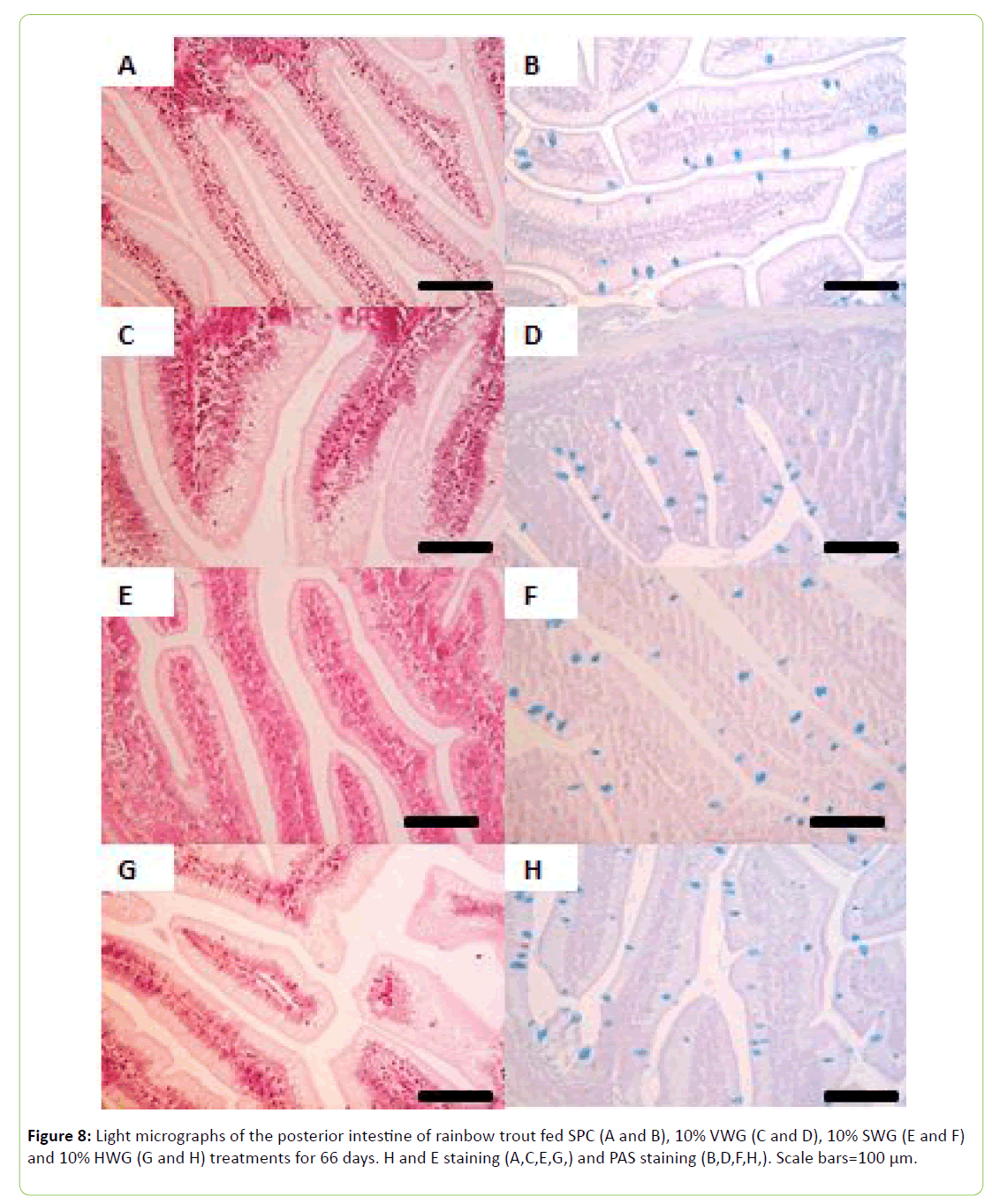
Light microscopy of the posterior intestine was carried out on two fish per tank (n=6 per treatment) after 24 hours starvation. Samples were fixed in 10% formalin for 24 hours and stored in 70% ethanol. Samples were dehydrated through a graded ethanol series and embedded in paraffin wax utilizing a Leica EG1150H tissue processor. Samples were sectioned at 5 μm utilizing a microtome (Leica RM2235) and stained with both haematoxylin and eosin (HandE) and periodic acid-Schiff with Alcian Blue (PAS).ks, UK). Images were captured utilizing a Leica DMIRB microscope mounted with an Olympus E410 digital SLR camera. Image analysis was carried out with the aid of Image J 1.45 (National Institutes of Health, USA). Goblet cells and intraepithelial leukocytes (IELs) were counted along measured entire intestinal folds as cells per 100 μm.
Statistical analysis
Data are presented as means ± standard deviation (SD), unless otherwise stated. Statistical analyses were carried out using Minitab 16 (Minitab 16 statistical software, Minitab Inc. State college Pennsylvania, USA). Growth performance data was analysed utilising ANOVA and Tukey’s post hoc test. Significance was accepted at P<0.05.
Alpha diversity matrices, Chao1 and Shannon’s diversity index for high throughput sequencing results were calculated through QIIME, and rarefied OTU tables to calculate Good’s coverage, assessing sampling depth coverage via observed genera. Intersample beta diversity analyses (metrics) were calculated using weighted unique fraction metric (UniFrac) distances and Bray- Curtis similarity. Statistical analysis was applied to sequences representing>0.1% of any treatment. Non parametric Kruskal- Wallis was followed with the post-hoc Tukey-Kramer test, performed using STAMP v2 0.8. Significance was accepted at P ≤ 0.05. A principal component analysis was carried out using R software (R. software, library FactoMineR; [13]) for gene expression parameters. The diet was added into the analysis as a supplementary qualitative variable.
Results
Growth performance
Growth performance; FCR, SGR, mean fish weight, PER, K factor and survival are presented in Table 3. Rainbow trout accepted all treatments and showed a good growth performance, with FCR’s ranging from 0.97 ± 0.07 in the 10% VWG diet to 1.10 ± 0.08 in the 10% SWG diet. FCR, SGR, PER, final average body weight, K factor and survival were unaffected by diets.
| SPC | 10% VWG | 10% HWG | 10% SWG | |
|---|---|---|---|---|
| FCR | 0.99 ± 0.03 | 0.97 ± 0.07 | 1.07 ± 0.06 | 1.10 ± 0.08 |
| SGR (%) | 1.65 ± 0.08 | 1.69 ± 0.11 | 1.59 ± 0.04 | 1.60 ± 0.06 |
| Final fish weight (g) | 70.24 ± 3.05 | 72.89 ± 6.62 | 70.50 ± 2.61 | 68.10 ± 1.77 |
| Protein efficiency ratio PER | 2.10 ± 0.05 | 2.13 ± 0.14 | 1.91 ± 0.10 | 1.89 ± 0.14 |
| K factor | 1.409 ± 0.06 | 1.421 ± 0.05 | 1.406 ± 0.12 | 1.387 ± 0.07 |
| Survival (%) | 95.83 ± 1.80 | 95.83 ± 1.80 | 89.58 ± 7.22 | 88.54 ± 11.83 |
Table 3: Growth performance of rainbow trout post 66 day feed trial.
High-throughput sequencing
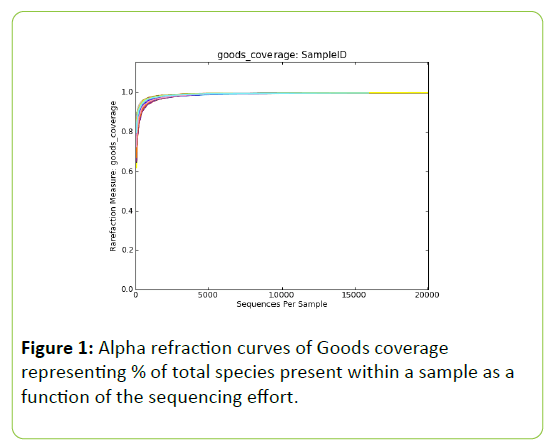
Post trimming and quality control 1,156,465 reads were retained for downstream analysis, identifying 1,064 distinct OTU’s. Alpha refraction analysis of goods coverage reveals estimations of>0.989 for the total species present per sample. Refraction of Goods coverage plateaued after approx. 5,000 reads per sample (Figure 1), suggesting that the bacterial communities were fully sampled and data are representative of the population. Alpha diversity parameters can be found in Table 4. Phylogenetic distances were significantly different between SWG and VWG, those obtained for SPC and HWG being intermediate. PCOA analysis revealed two main clusters. The first cluster consisting of the HWG and SWG diets and the second cluster of the VWG and the SPC diets. Two samples, 10% HWG replicate #6 and 10% SWG replicate #6, are distinct from both clusters.
| Treatment | Goods coverage | Observed species | Chao1 index | Shannon index | Phylogenetic distance |
|---|---|---|---|---|---|
| SPC | 0.993 ± 0.001 | 627.33 ± 93.87 | 811.84 ± 71.64 | 5.24 ± 0.78 | 22.78 ± 3.11ab |
| 10% VWG | 0.993 ± 0.02 | 688.66 ± 93.87 | 844.53 ± 81.86 | 5.82 ± 0.66 | 24.42 ± 3.22a |
| 10% HWG | 0.989 ± 0.004 | 567.16 ± 93.87 | 742.67 ± 88.7 | 5.64 ± 1.26 | 20.7 ± 2.91ab |
| 10% SWG | 0.99 ± 0.002 | 555.16 ± 93.87 | 747.06 ± 62.01 | 5.54 ± 0.67 | 19.67 ± 1.19b |
Table 4: High throughput sequencing alpha diversity parameters, goods coverage estimations by treatment and phylogenetic distance of the allochthonous bacterial communities in the posterior intestine of rainbow trout post 66 day feeding trial
The Firmicutes accounted for 76.17% of the total read sequences of all treatments (Figure 2). The Bacteroidetes were the next most dominant phyla (11.16%), followed by the Fusobacteria (4.32%), Proteobacteria (4.08%), Actinobacteria (1.50%), reads from the kingdom bacteria (phyla unknown) (1.50%), and the Chloroflexi (0.82%). Other phyla present in the sample-set, each with less than 0.2% of the total reads per phyla, combined accounted for 0.45%. Reads associated with the “kingdom:bacteria”, but of unknown phyla, accounted for an elevated percentage of the treatment reads of fish fed the 10% HWG (7.27% ± 6.61) compared to all other diets. The proportion of all other phyla was unaffected by dietary treatment Table 5.
| Taxon | SPC | 10% VWG | 10% HWG | 10% SWG |
|---|---|---|---|---|
| Phyla | ||||
| Actinobacteria | 1.32 ± 0.65 | 1.78 ± 1.59 | 1.12 ± 0.73 | 0.59 ± 0.18 |
| Bacteroidetes | 17.7 ± 14.92 | 10.59 ± 10.9 | 4.54 ± 6.2 | 23.56 ± 22.07 |
| Chloroflexi | 0.11 ± 0.05 | 0.13 ± 0.06 | 0.12 ± 0.09 | 0.1 ± 0 |
| Firmicutes | 76.62 ± 15.29 | 80.63 ± 10.89 | 66.27 ± 27.97 | 69.52 ± 24.25 |
| Fusobacteria | 1.66 ± 0.52 | 1.77 ± 0.22 | 13.63 ± 25.13 | 2.49 ± 0.55 |
| kingdom - Bacteria | 0.3 ± 0.12b | 1.59 ± 1.92b | 7.27 ± 6.61a | 0.77 ± 0.87b |
| Proteobacteria | 2 ± 1.25 | 3.05 ± 2.1 | 6.05 ± 6.45 | 2.56 ± 1.87 |
| Genus | ||||
| Ardenscatena | 0.08 ± 0.02 | 0.09 ± 0.01 | 0.11 ± 0.09 | 0.1 ± 0 |
| Bacillus | 5.73 ± 3.97b | 11 ± 8.05b | 2.37 ± 1.16b | 25.02 ± 12.28a |
| Bacteroides | 12.1 ± 10.17 | 7.23 ± 7.39 | 3.02 ± 4.2 | 16.24 ± 15.12 |
| Bradyrhizobium | 0.41 ± 0.69 | 0.37 ± 0.34 | 0.25 ± 0.11 | 0.19 ± 0.11 |
| Cetobacterium | 1.24 ± 0.39 | 1.48 ± 0.19 | 13.28 ± 25.14 | 1.74 ± 0.3 |
| Class - Alphaproteobacteria | 0 | 0 ± 0 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Class - Bacilli | 0.29 ± 0.11b | 0.14 ± 0.03b | 0.4 ± 0.27ab | 0.91 ± 0.65a |
| Enterococcus | 54.54 ± 21.08a | 38.79 ± 19.03ab | 13.74 ± 5.12b | 16.65 ± 6.38b |
| Facklamia | 0.45 ± 0.47 | 0.44 ± 0.35 | 0.88 ± 0.63 | 0.22 ± 0.08 |
| Family - Bacillaceae | 0.73 ± 0.5b | 1.12 ± 0.74b | 0.28 ± 0.06b | 5.57 ± 2.68a |
| Family - Enterobacteriaceae | 0.26 ± 0.23 | 0.14 ± 0.12 | 0.17 ± 0.22 | 0.52 ± 0.73 |
| Family - Enterococcaceae | 1.39 ± 0.59 | 1.08 ± 0.7 | 0.39 ± 0.14 | 0.41 ± 0.16 |
| Family - Leuconostocaceae | 0.05 ± 0.05 | 0.07 ± 0.03 | 0.11 ± 0.08 | 0.03 ± 0.01 |
| Family - Propionibacteriaceae | 0.03 ± 0.01 | 0.11 ± 0.07 | 0.17 ± 0.18 | 0.06 ± 0.01 |
| Family - Ruminococcaceae | 0.36 ± 0.09b | 2.38 ± 2.92ab | 12.42 ± 11.96a | 1.5 ± 2.09b |
| Family - Streptococcaceae | 0.21 ± 0.15 | 0.98 ± 0.72 | 0.79 ± 0.67 | 0.28 ± 0.16 |
| Fusobacterium | 0.29 ± 0.24 | 0.16 ± 0.09 | 0.16 ± 0.1 | 0.59 ± 0.82 |
| Gallicola | 0.53 ± 0.63 | 0.34 ± 0.12 | 0.42 ± 0.23 | 0.33 ± 0.08 |
| Kingdom - Bacteria | 0.3 ± 0.12b | 1.59 ± 1.92ab | 7.28 ± 6.62a | 0.77 ± 0.88b |
| Lactobacillus | 0.96 ± 0.75 | 2.17 ± 1.42 | 2.36 ± 1.87 | 0.75 ± 0.3 |
| Lactococcus | 0.15 ± 0.1 | 0.15 ± 0.1 | 0.61 ± 0.66 | 0.13 ± 0.04 |
| Leuconostoc | 0.42 ± 0.2b | 1.36 ± 0.78b | 7.68 ± 6.68a | 0.5 ± 0.13b |
| Macrococcus | 1.26 ± 0.56b | 0.52 ± 0.09b | 2.6 ± 2.38b | 8.23 ± 5.6a |
| Order - Bacillales | 1.32 ± 0.87b | 1 ± 0.49b | 1.06 ± 0.87b | 3.31 ± 1.44a |
| Order - Clostridiales | 0.02 ± 0 | 0.05 ± 0.03 | 0.15 ± 0.15 | 0.03 ± 0.01 |
| Order - Rhizobiales | 0.14 ± 0.11 | 0.43 ± 0.71 | 0.7 ± 1.33 | 0.12 ± 0.07 |
| Peptostreptococcus | 2.78 ± 4.17 | 0.62 ± 0.19 | 1.28 ± 1.51 | 0.61 ± 0.14 |
| Photobacterium | 0.08 ± 0.03 | 0.09 ± 0.02 | 0.13 ± 0.03 | 0.1 ± 0.02 |
| Phylum - Firmicutes | 0.21 ± 0.07 | 0.35 ± 0.07 | 1.33 ± 1.5 | 0.61 ± 0.73 |
| Psychrilyobacter | 0.09 ± 0.04 | 0.1 ± 0.02 | 0.14 ± 0.04 | 0.1 ± 0.02 |
| Rummeliibacillus | 0.19 ± 0.06b | 3.03 ± 2.3a | 0.19 ± 0.05b | 0.26 ± 0.1b |
| Staphylococcus | 0.92 ± 0.81 | 0.41 ± 0.17 | 0.92 ± 0.91 | 0.77 ± 0.37 |
| Weissella | 1.06 ± 0.54b | 11.75 ± 6.26a | 9.4 ± 8.55ab | 0.99 ± 0.27b |
Table 5: Allochthonous bacterial communities in the posterior intestine of rainbow trout post 66 day feeding with experimental diets. Data are represented as phyla and genus percentage means ± SD. Data excludes phyla and genus with less than 0.2% of the total reads. Kruskal-Wallis with post hoc Tukey-Kramer. Significance accepted at P<0.05.
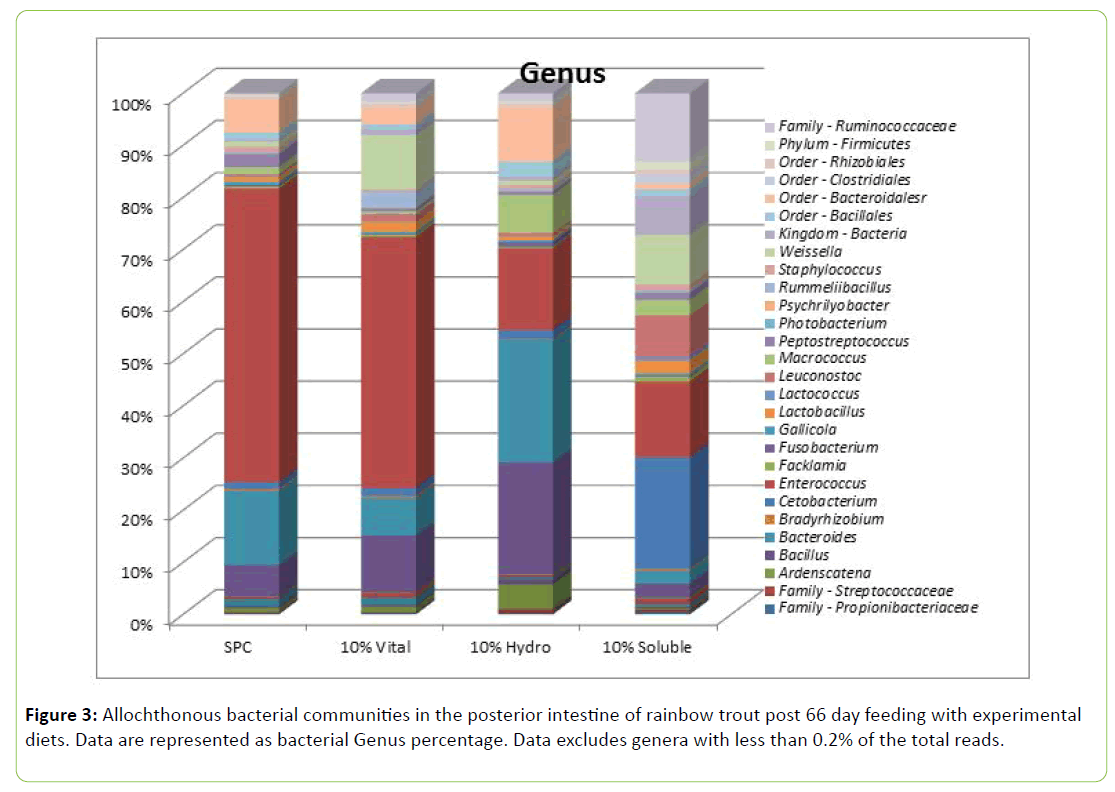
It can however be noticed that the relative abundance of Firmicutes was numerically lower with SWG and HWG diets. The sequence distribution data a genus level is displayed in Figure 3. The most abundant genus was Enterococcus, representing 46.52% of the total reads. Bacteroides represented the next most abundant genus (7.65%) followed by Bacillus (6.66%), order: Bacteroidales (genus unknown) (3.49%), Weissella (3.43%), Cetobacterium (3.36%), family: Ruminococcaceae (genus unknown) (2.21%), Peptostreptococcus (1.99%) and Macrococcus (1.60%). The remaining genera present represent <1.5% of total reads.
Enterococcus, the most abundant genus as a percentage of total reads was significantly (P<0.05) elevated in the 10% VWG and SPC fed fish (38.79% ± 19.03 and 54.54% ± 21.08, respectively) compared to the 10% HWG and 10% SWG fed fish (13.74% ± 5.12 and 16.65% ± 6.38 respectively; Table 6). Bacillus was more abundant in the 10% SWG diet; while Leuconostoc was more abundant in the HWG diet than in the other diets (P<0.05). Weissella was more abundant in the 10% VWG and intermediate with HWG.
| Correlation coefficient | P-value | % cumulative variance | |
|---|---|---|---|
| Dimension 1 | 44.6 | ||
| TNF-a | 0.82 | <0.001 | |
| IL10 | 0.81 | <0.001 | |
| TGF-β | 0.76 | <0.001 | |
| Glute ST | 0.57 | 0.009 | |
| IL8 | 0.49 | 0.027 | |
| HSP70 | 0.46 | 0.041 | |
| Qualitative: SPC diet | Estimate: 1.62 | 0.008 | |
| Dimension 2 | 69.2 | ||
| IL8 | 0.71 | <0.001 | |
| HSP70 | 0.59 | 0.007 | |
| IL10 | -0.51 | 0.023 | |
| TGF-β | -0.56 | 0.01 | |
| Qualitative: SWG | Estimate: -1.01 | 0.031 | |
| Dimension 3 | 83.8 | ||
| Glute ST | 0.76 | <0.001 | |
| Correlation coefficient | P-value | % cumulative variance | |
| Dimension 1 | 44.6 | ||
| TNF-a | 0.82 | <0.001 | |
| IL10 | 0.81 | <0.001 | |
| TGF-β | 0.76 | <0.001 | |
| Glute ST | 0.57 | 0.009 | |
| IL8 | 0.49 | 0.027 | |
| HSP70 | 0.46 | 0.041 | |
| Qualitative: SPC diet | Estimate: 1.62 | 0.008 | |
| Dimension 2 | 69.2 | ||
| IL8 | 0.71 | <0.001 | |
| HSP70 | 0.59 | 0.007 | |
| IL10 | -0.51 | 0.023 | |
| TGF-β | -0.56 | 0.01 | |
| Qualitative: SWG | Estimate : -1.01 | 0.031 | |
| Dimension 3 | 83.8 | ||
| Glute ST | 0.76 | <0.001 |
Table 6: Three first dimensions of the principal component analysis performed on gene expression parameters.
Gene expression
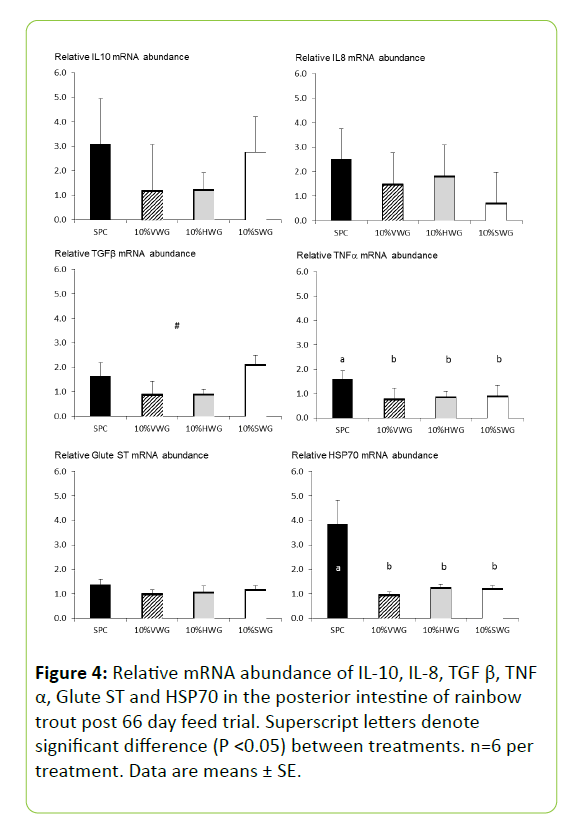
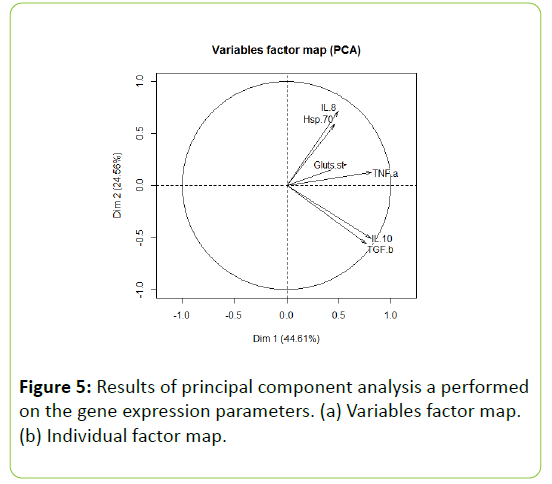
The relative expressions of the pro-inflammatory cytokine IL-8, the anti-inflammatory cytokine IL-10, and Glute-ST were unaffected by dietary treatment (Figure 4). The immuneregulatory cytokine TGF-β tended to be lower with VWG and HWG than with SPC and SWG (P=0.109). The pro-inflammatory cytokine TNF-α and HSP70 expression were significantly downregulated in all wheat gluten treatments compared to the SPC diet (P=0.045 and P<0.001 respectively; Figure 4). Furthermore, the PCA revealed that the three main axes allowed explaining around 83.8% of the variance. The relative abundance of TNF-α, IL10, TGF-β, Glute ST, IL8 and HSP70 were characterized the first dimension (Table 6). This dimension was significantly associated with SPC diet (P=0.008; Figure 5). The second dimension was positively correlated with the relative abundance of IL8 and HSP70; while negatively with the relative abundance of IL10 and TGF-β. The dimension was negatively associated with the SWG diet (P=0.03; Table 6). The third dimension was positively correlated with the relative abundance of Glute ST.
Intestinal histology
Electronic microscopy
Scanning electron micrographs of the posterior intestine of the rainbow trout revealed no qualitative effects induced by the inclusion of wheat gluten in the experimental diets compared to the SPC treatment (Figure 6). Gross structure qualitative analysis showed evenly shaped and distributed enterocytes with no signs of necrosis or gross damage. Microvilli form and distribution looked uniform across treatments with densely packed microvilli, with no sign of patchy or damaged areas. Quantitative analysis of microvilli density (arbitrary units) was also unaffected by the dietary regimes fed to experimental fish, despite numerical trends (Figure 7).
Light microscopy
Goblet cell counts conducted on the PAS stained sections revealed no significant difference (P>0.05; Figure 8) between treatments. Intraepithelial leukocytes, per 100 um-1, were significantly higher in the 10% VWG and 10% HWG treatments (0.45 ± 0.03 and 0.44 ± 0.03, respectively) compared to the SPC treatment (0.34 ± 0.02) (P<0.05). Significant difference between treatments was also observed in lamina propria width being higher in VWG and HWG-fed fish compared to those fed SPC diet (Table 7).
| SPC | 10% VWG | 10% HWG | 10% SWG | |
|---|---|---|---|---|
| Goblet cells (100 µm-1) | 1.41 ± 0.06 | 1.49 ± 0.05 | 1.57 ± 0.07 | 1.33 ± 0.05 |
| Intraepithelial leukocytes (100 µm-1) | 0.34 ± 0.02b | 0.45 ± 0.03a | 0.44 ± 0.03a | 0.38 ± 0.03ab |
| Lamina propria width (µm) | 9.48 ± 0.41a | 11.83 ± 0.44b | 11.03 ± 0.36b | 10.29 ± 0.35ab |
Table 7: Histological parameters of the posterior intestine of rainbow trout fed experimental diet for 66 days. Data are means ± SE. significance indicated by superscript letters accepted at P<0.05.
Discussion
Due to the low price and large production, plant proteins have been and will probably continue being the main choice to replace fishmeal. However plant proteins are diverse, can exhibit high levels of anti-nutritional factors or supply inadequate amounts of nutrients, notably amino acids. For salmonids, a number of studies have shown that a large proportion of fishmeal could be replaced by plant ingredients without deleterious effects; while other reported that replacing fishmeal resulted in decreased growth performance [14]. Low palatability of plant-proteins is considered as a major constraint leading to lower feed intake. Furthermore, independently to the effect on feed intake, high levels of plant proteins can reduce the feed efficiency, pointing towards a digestive or metabolic problem. Studies with salmonids showed that plant-proteins as soybean meal [11], and pea protein concentrate [15] may provoke morphological changes and inflammation in the distal intestine. Overall, there is a poor understanding of the physiological reactions of fish to specific plant proteins; and further investigations are required to optimize feed formulation.
Juvenile rainbow trout fed low fishmeal and high soy-protein diets performed well throughout the trial, with FCR’s values lower than 1 meaning that juvenile rainbow trout showed a good growth performance and feed utilization with high level of plant proteins. FCR and SGR values are in agreement or even better than those obtained [16] who used solvent extracted cottonseed meal from 0 to 61% inclusion rate at the expense of fishmeal in diets for juvenile rainbow trout with an initial body weight gain of 39 g at temperature of 16°C [17]. Obtained SGR of 1.66 in rainbow trout having an initial body weight of 38.5 g and fed diets with 27% fishmeal, 8% soybean meal and 45% of co-extruded product of rapeseed and peas. Our study confirmed that juvenile rainbow trout can grow well when feeding diets containing only 10% fishmeal.
Wheat proteins inclusions had no significant effect on FCR, SGR, PER. Different data suggested that wheat gluten is a good protein source for salmonids, and more widely for carnivorous fish at different stages of the life, reared in salted or fresh water. Good growth performance has been observed when using wheat gluten as a replacement of both fish meal in Atlantic salmon (Salmo salar) [18] and plant based proteins in rainbow trout [19]. Interestingly, vital wheat gluten was used in combination with potato protein concentrate in diets containing 26.5% fishmeal for rainbow trout weighting 52.2 g at the beginning and reared in fresh water at 13.4°C [19]. They tested different inclusion rates of both potato protein concentrate and wheat gluten and observed no significant difference in growth performance compared to the fishmeal-based diet. Improved weight gain has been reported in rainbow trout with wheat gluten replacement of fishmeal and soy-protein with additional amino-acid supplementation [20]. Overall, these data suggest high nutritional values of vital wheat gluten.
No difference between the wheat proteins types have been obtained on growth performance. Only few studies evaluated the effects of HWG, and no study aimed at comparing the effects of the wheat protein type i.e., vital or hydrolyzed, in fish. Storebakken et al. [8] observed improved protein apparent digestibility coefficients for HWG compared to fishmeal diet. Furthermore, decreased FCR’s have been observed in juvenile hybrid sturgeon (Acipenser schrenckii ÃÆâÃâââ¢Ãâââ¬ × Huso dauricus ÃÆâÃâââ¢Ãâââ¬Å¡) fed with 5% HWG in replacement to soy-protein concentrate [21]; while an addition of 6% HWG in diets of juvenile barramundi (Lates calcarifer) resulted in better feed efficiency [9]; suggesting that HWG is a good protein source. In the current study, we failed to highlight significant differences between the wheat protein types on growth performance. The substitution of fishmeal by VWG or HWG in weaning pig diets did not affect pig growth up to 5 weeks after weaning. Interestingly, VWG and HWG diets had higher apparent digestibilities, maybe as a result of a reduced negative physiological impact of weaning on the enteric mucosa. However, there were no differences in productive performance that might be attributed to the enhanced protein solubility of the HWG [22]. In another study performed on broilers [23], FCR was significantly higher with a HWG diet than with a VWG, suggesting a better availability of amino-acid with hydrolysation. One explanation of the absence of effect in our study could be the positive performance of all treatments including the SPC and VWG treatments, with a FCR lower than 1 that might have masked a possible beneficial effect of HWG or SWG inclusions.
Effects of wheat proteins on gut microbiota
Gut microbiota of fish has been investigated to evaluate how the inclusion of wheat proteins in high soy-protein diets may impact microbial populations. Alpha refraction analysis of Good’s coverage reveals estimations of >98.9%, indicative of a fully sampled microbiome. The sequence distribution data reads of 16S rRNA was dominated by the Firmicutes at phylum level, accounting for 76.17% of the total read sequences of all treatments, in line with the observations of Mansfield et al. [24] when increasing plant protein source inclusions. The Bacteroidetes were the second most dominant phylum (11.16%), followed by the Fusobacteria (4.32%), Proteobacteria (4.08%), Actinobacteria (1.50%) and Chloroflexi (0.82%). The proportion of identified phyla was unaffected by dietary treatment and have previously been described as part of the commensal intestinal bacteria of fish [25-30].
The impact on gut microbiota differed according to the wheat proteins type. Primarily, even if the result was not significant, a shift between Firmicutes and Bacteroidetes was observed with SWG and HWG compared to VWG and SPC diets. In line with this primary observation, two clusters were identified, the first cluster consisting of the HWG and SWG diets and the second cluster of the VWG and the SPC diets. At genera level, growth of different potentially probiotic species according to wheat proteins type was revealed. Bacillus, class Bacilli, was significantly elevated in the SWG diet. Of the Bacillus reads identified by high throughput sequencing, the majority belonged to B. coagulans. The probiotic potential of B. coagulans has been investigated in common, grass and koi carp (Cyprinus carpio, Ctenopharyngodon idella and Cyprinus carpio koi, respectively [31-33]. The relative abundance of Weissella was also modulated as it significantly increased in the VWG diet and tended to increase with HWG diet. Weissella has been routinely identified in the intestines or of carnivorous fish, including salmonids [9,24,27,34,35]. Of the Weissella reads identified by high throughput sequencing, the majority belonged to Weissella confusa. W. confusa has been identified as a potential probiotic species for juvenile Asian sea bass [36], with no information to the author’s knowledge on its effects on salmonids. Apper et al. [9] observed significantly increased W. confusa reads with 6% HWG inclusion in a commercial recipe. Overall, the use of wheat products could be useful to favour growth of potential beneficial bacteria. However, further research allowing correlations between the relative abundance of this potential bacteria and health and growth markers will be required to confirm this hypothesis.
Effects of wheat proteins on gut non-specific immune parameters and on gut morphology
The health and structure of the intestine of fish is of high importance as the gastrointestinal tract plays a key role in digestive function and the immune system, acting as a first line of defence to many water borne fish pathogens. In our study, wheat proteins have a positive effect on localized immunological status of rainbow trout fed a high soy-protein diet, evaluated by the relative transcriptional expression levels of a number of immunologically relevant genes. HSP70, known to mediate the degradation and repair of denatured or altered proteins, is highly conserved at amino acid level [37,38]. It was significantly reduced in all wheat proteins diets compared the SPC control. Levels of expression are known to be affected by various stressors, such as pathogen invasion and detrimental stocking densities [39-41]. A significant up-regulation of HSP70 has been observed in the posterior intestine of Atlantic salmon (Salmo salar) fed soybean meal based diets, compared to fishmeal controls, which is attributed to elevated cellular stress and subsequent increased cellular repair mechanisms and apoptosis [42]. The decrease in TNF-a observed with all types of wheat proteins vs. SPC also supports elevated cellular stress. In addition, the fact that the PCA analysis revealed that SPC group is characterized by high relative abundance of IL8, HSP70 and TNF-a and was different from the SWG diet characterized by high IL10 and TGF-β relative abundances are in agreement with the previous findings. Overall, it can therefore be inferred that wheat proteins have a reduced cellular stressor fraction than soya proteins, potentially due to their lower concentrations of ANFs, or/and to the modulation of gut microbiota. Wheat proteins are thus high quality proteins to be added in low fishmeal diet in addition to various leguminous proteins known to contain high levels of ANFs.
Similarly, results obtained on gut morphology are in favour of a beneficial effect of wheat proteins. Scanning electron microscopy revealed no dietary effect on the microvilli density of enterocytes in the posterior intestine at the end point of the feeding trial. On the contrary, increased absorptive surface area has been observed in juvenile Asian seabass (Lates calcarifer) [9] as well as intestinal fold height/villous height in the intestine of hybrid sturgeon (A. schrenckii ÃÆâÃâââ¢Ãââ⬠x H. dauricus ÃÆâÃâââ¢Ãâââ¬Å¡) and broilers with dietary HWG included in both commercial and experimental diets [21,23]. Interestingly, in broilers, the effect on villi was observed with HWG but not with VWG when broilers were challenged with a large amount of pectin [23]. This result might suggest that the potential benefits of HWG on gut histo-morphology could be expressed when animals are challenged. While goblet cell abundance in the posterior intestine was not affected by dietary treatments; laminia propria width and IEL numbers in the posterior intestine were significantly elevated in the wheat proteins diets treatments compared to the SPC diet. The lack of lymphoid structures in teleost intestinal folds enhances the importance of leukocytes in the epithelium and lamina propria as protection against pathogenic insult. Comprised of diffuse populations of phagocytes, natural cytotoxic cells and lymphocytes, changes in IEL’s populations can be interpreted in two manners. Firstly, as an increase in immune readiness for potential pathogen encounters, or secondly, an increase could be due to pathogen identification and an active immune response. The elevated IEL numbers observed in the study combined with the high level of performance and gene expression analysis of inflammatory cytokines IL-8 and TGF-β, associated with leukocyte chemotaxis, would suggest there is no underlying pathogenic insult. Due to the lack of evidences to prove a pathogenic insult, it could be concluded that the 10% inclusion of wheat proteins enhances the immune potential of the posterior intestine.
One limitation of our study was that diets contained huge amount a soy products, so that the observed effects could be more related to the large amount of soy proteins than to the inclusion of the wheat proteins. However, we performed another trial with the same soy protein-based control and with up to 30% wheat proteins, and we observed significant increase in FCR and SGR and a stimulation of the growth of Weissella confusa when comparing to the control diet [43]. Furthermore, a significant improvement of FCR as well as an increase in the absorption area and a stimulation of the growth of Weissella confusa were obtained when including 6% of HWG at the expense of all other protein sources in juvenile barramundi (Lates calcarifer) fed a commercial diet [9]. Taking together, these results exhibited that the positive effects were directly related to wheat proteins, and not to the decrease in soy protein concentrate.
Conclusion
In conclusion, wheat gluten products can be included at a 10% in soy-based diets to replace fishmeal. All wheat protein types provided adequate growth performance with the added benefits of enhancing the growth of population of beneficial bacterial genera in the posterior intestine, reducing expression of different markers of intestinal stress and potentially enhancing the non-specific innate immune system of rainbow trout. HWG and SWG seem to modulate gut microbiota in a different way than SPC and VWG diets. To the author’s knowledge there is no previous literature on the effect of different wheat gluten products on the localized intestinal immunity of fish in low fishmeal high pulse diet. Wheat proteins are added-value proteins for alleviating possible negative effects of pulse proteins on gut health and microbiota. Further investigations are needed to clarify the effect of wheat proteins in commercial trout diets. Other experiments in a more challenging environment or with fish deleterious immune conditions are also needed to evaluate the benefits of wheat proteins on gut microbiota, morphology and non-specific immune parameters in deleterious conditions as an immune or a temperature challenge.
Funding
The study was supported by the company Tereos Starch and Sweeteners Europe. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests
This study received funding from Tereos Starch and Sweeteners Europe a company producing wheat proteins belonging to the company ‘‘Tereos’’. EA is employed by Tereos. There are no further patents, products in development or marketed products to declare. This does not alter the authors’ adherence to all the Aquaculture policies on sharing data and materials.
Contributors
Conceived and designed the experiments: SV EA DM. Performed the experiments: SV AR DM. Analyzed the data: SV EA AR DM. Wrote the paper: SV EA AR DM. All authors have approved the final article.
References
- Alexis MN (1997) Fish meal and fish oil replacers in Mediterranean marine fish diets. Feeding tomorrow's fish, Zaragoza: CIHEAM pp: 183-204
- Espe M, Lemme A, Petri A, El-Mowafi A (2006) Can Atlantic salmon (Salmo salar) grow on diets devoid of fish meal? Aquac 255: 255-262.
- Carter CG, Hauler RC (2000) Fish meal replacement by plant meals in extruded feeds for Atlantic salmon, Salmo salar L. Aquac 185: 299-311.
- Hardy RW (2010) Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquac Res 41: 770-776.
- Burr GS, Wolters WR, Barrows FT, Hardy RW (2012) Replacing fishmeal with blends of alternative proteins on growth performance of rainbow trout (Oncorhynchus mykiss), and early or late stage juvenile Atlantic salmon (Salmo salar). Aquac 334-337: 110-116.
- Tacon AGJ, Metian M (2008) Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquac 285: 146-158.
- Apper-Bossard E, Feneuil A, Wagner A, Respondek F (2013) Use of vital wheat gluten in aquaculture feeds. Aquat Biosyst 9: 21-34.
- Storebakken T, Zhang Y, Ma J, Øverland M, Mydland LT, et al. (2015) Feed technological and nutritional properties of hydrolyzed wheat gluten when used as a main source of protein in extruded diets for rainbow trout (Oncorhynchus mykiss). Aquac 448: 214-218.
- Apper E, Weissman D, Respondek F, Guyonvarch A, Baron F, et al. (2016) Hydrolysed wheat gluten as part of a diet based on animal and plant proteins supports good growth performance of Asian seabass (Lates calcarifer), without impairing intestinal morphology or microbiota. Aquac 453: 40-48.
- Salinas I, Parra D (2015) Fish mucosal immunity: Intestine A2-Beck, Benjamin H. In: PEATMAN, E. (ed.) Mucosal Health in Aquaculture. San Diego: Academic Press.
- Merrifield DL, Dimitroglou A, Bradley G, Baker RT, Davies SJ, et al. (2009) Soybean meal alters autochthonous microbial populations, microvilli morphology and compromises intestinal enterocyte integrity of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 32: 755-66.
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3: RESEARCH0034.
- Cornillon PA, Guyader A, Husson F, Jégou N, Josse J, et al. (2008) Analyse factorielle des correspondances. In Statistiques avec R (eds E Mattzner-Lober), 190-196. Presse Universitaire de Rennes, Rennes, France.
- Hua K, Bureau DP (2012) Exploring the possibility of quantifying the effcets of plant protein ingredients in fish feeds using meta-analysis and nutriitonal model simulation-based approaches. Aquac 356-357: 284-301.
- Penn MH, Bendiksen EA, Campbell P, Krogdahl A (2011) High level of dietary pea protein concentrate induces enteropathy in Atlantic salmon (Salmo salar L.). Aquac 310: 267-273.
- Luo L, Xue M, Wu X, Cai X, Cao H, et al. (2006) Partial or total replacement of fishmeal by solventÃÆâÃâââ¬ÃâÃÂextracted cottonseed meal in diets for juvenile rainbow trout (Oncorhynchus mykiss). Aquac Nutr 12: 418-424.
- Gomes EF, Corraze G, Kaushik S (1993) Effects of dietary incorporation of a co-extruded plant protein (rapeseed and peas) on growth, nutrient utilization and muscle fatty acid composition of rainbow trout (Oncorhynchus mykiss). Aquac 113: 339-353.
- Storebakken T, Shearer KD, Baeverfjord G, Nielsen BG, Åsgård T, et al. (2000) Digestibility of macronutrients, energy and amino acids, absorption of elements and absence of intestinal enteritis in Atlantic salmon, Salmo salar, fed diets with wheat gluten. Aquac 184: 115-132.
- Tusche K, Arning S, Wuertz S, Susenbeth A, Schulz C, et al. (2012) Wheat gluten and potato protein concentrate-Promising protein sources for organic farming of rainbow trout (Oncorhynchus mykiss). Aquac 344-349: 120-125.
- Davies SJ, Morris PC, Baker RTM (1997) Partial substitution of fish meal and full-fat soya bean meal with wheat gluten and influence of lysine supplementation in diets for rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Res 28: 317-328.
- Qiyou X, Qing Z, Hong X, Chang`An W, Dajiang S, et al. (2011) Dietary glutamine supplementation improves growth performance and intestinal digestion/absorption ability in young hybrid sturgeon (Acipenser schrenckii nchus mykiss. na ÃÆâÃâââ¢Ãâââ¬Å¡). J Appl Ichthyol 27: 721-726.
- Blasco M, Fondevila M, Guada JA (2005) Inclusion of wheat gluten as a protein source in diets for weaned pigs. Anim Res 54: 297-306.
- Van Leeuwen P, Mouwen JM, Van Der Klis JD, Verstegen MW (2004) Morphology of the small intestinal mucosal surface of broilers in relation to age, diet formulation, small intestinal microflora and performance. Br Poult Sci 45: 41-48.
- Mansfield GS, Desai AR, Nilson SA, Van Kessel AG, Drew MD, et al. (2010) Characterization of rainbow trout (Oncorhynchus mykiss) intestinal microbiota and inflammatory marker gene expression in a recirculating aquaculture system. Aquac 307: 95-104.
- Navarrete P, Toledo I, Mardones P, Opazo R, Espejo R, et al. (2010) Effect of Thymus vulgaris essential oil on intestinal bacterial microbiota of rainbow trout, Oncorhynchus mykiss (Walbaum) and bacterial isolates. Aquac Res 41: e667-e678.
- Wu S, Wang G, Angert ER, Wang W, Li W, et al. (2012) Composition, diversity, and origin of the bacterial community in grass carp intestine. PLoS ONE 7: e30440.
- Wong S, Waldrop T, Summerfelt S, Davidson J, Barrows F, et al. (2013) Aquacultured rainbow trout (Oncorhynchus mykiss) possess a large core intestinal microbiota that is resistant to variation in diet and rearing density. Appl Environ Microbiol 79: 4974-4984.
- Dehler CE, Secombes CJ, Martin SAM (2016) Environmental and physiological factors shape the gut microbiota of Atlantic salmon parr (Salmo salar L). Aquac 467: 149-157.
- Gajardo K, Rodiles A, Kortner TM, Krogdahl Å, Bakke AM, et al. (2016) A high-resolution map of the gut microbiota in Atlantic salmon (Salmo salar): A basis for comparative gut microbial research. Sci Rep 6: 30893.
- Michl SC, Ratten JM, Beyer Hasler M, LaRoche J (2017) The malleable gut microbiome of juvenile rainbow trout (Oncorhynchus mykiss): Diet-dependent shifts of bacterial community structures. PlosOne 12: e0177735.
- Wang Y (2011) Use of probiotics Bacillus coagulans, Rhodopseudomonas palustris and Lactobacillus acidophilus as growth promoters in grass carp (Ctenopharyngodon idella) fingerlings. Aquac Nutr 17: e372-e378.
- Lin S, Mao S, Guan Y, Luo L, et al. (2012) Effects of dietary chitosan oligosaccharides and Bacillus coagulans on the growth, innate immunity and resistance of koi (Cyprinus carpio koi). Aquac 342-343: 36-41.
- Xu Y, Wang Y, Lin J (2014) Use of Bacillus coagulans as a dietary probiotic for the common carp, Cyprinus carpio. J World Aquac Soc 45: 403-411.
- Hovda MB, Fontanillas R, Mcgurk C, Obach A, Rosnes JT, et al. (2012) Seasonal variations in the intestinal microbiota of farmed Atlantic salmon (Salmo salar L.). Aquac Res 43: 154-159.
- Reveco FE, Øverland M, Romarheim OH, Mydland LT (2014) Intestinal bacterial community structure differs between healthy and inflamed intestines in Atlantic salmon (Salmo salar L.). Aquac 420-421: 262-269.
- Rengpipat S, Rueangruklikhit T, Piyatiratitivorakul S (2008) Evaluations of lactic acid bacteria as probiotics for juvenile seabass Lates calcarifer. Aquac Res 39: 134-143.
- Kiang JG, Tsokos GC (1998) Heat shock protein 70 kDa: Molecular biology, biochemistry, and physiology. Pharmacol Ther 80: 183-201.
- Basu N, Todgham AE, Ackerman PA, Bibeau MR, Nakano K, et al. (2002) Heat shock protein genes and their functional significance in fish. Gene 295: 173-183.
- Gornati R, Papis E, Rimoldi S, Terova G, Saroglia M, et al. (2004) Rearing density influences the expression of stress-related genes in sea bass (Dicentrarchus labrax L.). Gene 341: 111-8.
- Sanden M, Olsvik PA (2009) Intestinal cellular localization of PCNA protein and CYP1A mRNA in Atlantic salmon Salmo salar L. exposed to a model toxicant. BMC Physiol 9: 1-11.
- Zhang Q, Yu H, Tong T, Tong W, Dong L, et al. (2014) Dietary supplementation of Bacillus subtilis and fructooligosaccharide enhance the growth, non-specific immunity of juvenile ovate pompano, Trachinotus ovatus and its disease resistance against Vibrio vulnificus. Fish Shellfish Immunol 38: 7-14.
- Bakke-Mckellep AM, Penn MH, Salas PM, Refstie S, SperstadS, et al. (2007) Effects of dietary soyabean meal, inulin and oxytetracycline on intestinal microbiota and epithelial cell stress, apoptosis and proliferation in the teleost Atlantic salmon (Salmo salar L.). Br J Nutr 97: 699-713.
- Voller S, Rodiles A, Davies SJ, Apper E, Merrifield D, et al. (2016) Evaluation of dietary wheat gluten products and scFOS on gut health and growth performance of Rainbow trout (Oncorhynchus mykiss). In 67th Annual Meeting of the European Federatio of Animal Science, Belfast, UK.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences