The Relationship between Liver Lipid Accumulation and Changes in Plasma Amino Acids in Mice Challenged with Carbon Tetrachloride
Shinya Takagi, Daichi Oikawa, Hiromi Ikeda, Nozomi Tateiwa, Kazunori Koba, Vishwajit S. Chowdhury, Shinobu Yasuo, Mitsuhiro Furuse.
DOI10.21767/2572-5459.100012
Shinya Takagi1, Daichi Oikawa2, Hiromi Ikeda1, Nozomi Tateiwa3, Kazunori Koba3, Vishwajit S. Chowdhury4, Shinobu Yasuo1 and Mitsuhiro Furuse1*
1Laboratory of Regulation in Metabolism and Behavior, Faculty of Agriculture, Kyushu University, Fukuoka 812-8581, Japan
2Faculty of Education, Nagasaki University, Nagasaki 852-8521, Japan
3Department of Nutritional Science, University of Nagasaki, Siebold Nagasaki 851-2195, Japan
4Division for Experimental Natural Science, Faculty of Arts and Science, Kyushu University, Fukuoka 819-0395, Japan
- Corresponding Author:
- Mitsuhiro Furuse
Laboratory of Regulation in Metabolism and Behavior
Faculty of Agriculture, Kyushu University
Fukuoka 812-8581, Japan
E-mail: furuse@brs.kyushu-u.ac.jp
Received date: January 20, 2016, Accepted date: February 12, 2016, Published date: February 18, 2016
Citation: Takagi S, Oikawa D, Ikeda H, et al. The Relationship between Liver Lipid Accumulation and Changes in Plasma Amino Acids in Mice Challenged with Carbon Tetrachloride. J Anim Res Nutr 2016, 1:12. doi: 10.21767/2572-5459.100012
Copyright: © 2016 Takagi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Acute liver damage induced by the administration of carbon tetrachloride is characterized by lipid accumulation in the liver and changes in the plasma free amino acid profile. The major objective of the present study was to clarify the relationships between liver lipid levels and plasma free amino acids, and to estimate liver lipid contents via the concentrations of plasma free amino acids. Mice were administered with carbon tetrachloride or olive oil. Liver and plasma samples were obtained before (initial value) and after 1, 3 and 7 days of administration. Liver triacylglycerol increased at 1 day and total cholesterol was raised at 1 and 3 days post injection of carbon tetrachloride, and thereafter both returned to their initial values. L-Phenylalanine, L-serine and L-alanine were significantly increased, but L-arginine was significantly decreased at 1 day. Liver triacylglycerol and total cholesterol levels were linearly, but weakly, correlated with several amino acids. Thus, stepwise regression analysis was applied and we found that the liver triacylglycerol level could be estimated by the levels of free plasma L-histidine, L-methionine and L-phenylalnine, and that the liver total cholesterol could be estimated by the levels of L-glutamine, L-valine and L-phenylalnine. In conclusion, the increase in plasma free L-phenylalanine plays an important factor in the accumulation of liver lipid induced by acute treatment with carbon tetrachloride in mice.
Keywords
Carbon tetrachloride; Liver; Plasma amino acids; Total cholesterol; Triacylglycerol
Introduction
The liver acts as a vital organ for different metabolic activities, such as carbohydrate, lipid and protein metabolism, which are interrelated with one another. The interrelationships among these metabolic processes can be confirmed when there are injuries to the liver and/or abnormalities in it, when simultaneous disturbance in functions occurs. Liver cirrhosis most commonly occurs as a result of alcoholism, but some poisons such as carbon tetrachloride and viral diseases can also induce liver cirrhosis. Among poisons, carbon tetrachloride is widely used for experimental cirrhosis.
It is well known that acute administration of carbon tetrachloride modifies lipid metabolism, resulting in a fatty liver. Weber et al. [1] summarized an overview of the effects of carbon tetrachloride on lipid metabolism in the liver. Carbon tetrachloride increases: 1) the synthesis of fatty acids; 2) the synthesis of triacylglycerols from acetate; 3) the rate of lipid esterification; and 4) the synthesis of cholesterol; but lowers 1) β-oxidation of fatty acids and 2) hydrolysis of triacylglycerols.
In the case of protein metabolism, as a result of hepatic insufficiency due to carbon tetrachloride the plasma free amino acid levels were distinctly abnormal. Most of the amino acids except the branched chain amino acids (BCAAs) (i.e. valine, leucine and isoleucine) are metabolized in the liver. The BCAAs are mainly metabolized in the muscle. Among the amino acids metabolized in the liver, the increased plasma level of aromatic acids (AAA) such as phenylalanine and tyrosine is referred to as an indicator of liver disease [2]. Fischer et al. [3] proposed the ratio of the sum of the plasma BCAA levels divided by the sum of the plasma AAA levels, and this ratio was recognized as Fischer’s ratio. This ratio showed an excellent correlation with a grade of encephalopathy. According to Muratsubaki and Yamaki [4], not only plasma AAA but also most of the plasma amino acids other than arginine were significantly increased in rats through carbon tetrachloride treatment.
As mentioned above, carbon tetrachloride strongly influences lipid and amino acid metabolism in the liver. However, the relationships between accumulated levels of lipids in the liver and plasma free amino acid concentration have not yet been clarified. To clarify this issue, the correlation between liver lipid levels and plasma free amino acid levels in mice was determined after challenging the mice with carbon tetrachloride.
Materials and Methods
Animals and treatments
Six-week-old male ICR mice (Japan SLC, Shizuoka, Japan) were housed in conditions including room temperature at 22-23°C, humidity at 50-60% and a 12-h light-dark cycle (8:00-20:00); they were housed individually, and had free access to a commercial diet (MF, Oriental Yeast Co. Ltd., Tokyo, Japan) and water.
Mice were given free access to the commercial diet. After 8 days of acclimatization, the mice were divided into seven groups (n = 7) based on similar body weight. One group was assigned as the initial group without any treatments. Three groups were exposed to a carbon tetrachloride solution (2 ml/kg) and the other three groups were exposed to olive oil (2 ml/kg). The carbon tetrachloride solution consisted of the same volume of carbon tetrachloride and olive oil. The carbon tetrachloride solution and the olive oil were orally administered once on the day of treatment. Sampling of the initial group was done on the day of treatment of the other six groups. The treated groups were sampled at 1, 3 and 7 days post injection. The mice were killed by cervical dislocation and decapitation.
Experimental procedures followed the guidelines for animal experiments of the University of Nagasaki (No. 26-15).
Sample collection
Blood was collected from the carotid artery into tubes containing heparin sodium (AY Pharma, Tokyo, Japan) once the mice had been decapitated after cervical dislocation. To obtain a plasma sample, blood was centrifuged for 20 min at 850×g. The liver was then removed and weighed. The plasma and liver samples were stored at -80°C until the analysis of lipids and free amino acids took place.
Plasma amino acid assay
Each sample of plasma was filtered through an Amicon® Ultra centrifugal filter (0.5 ml 3K, Merck Millipore, USA) and centrifuged at 14,000×g for 15 min. Supernatants of 10 μl were used. Both the L- and the D-amino acid contents were measured by a UPLC system (the AcquityTM UPLC system was comprised of Waters Binary Solvent Manager, Water Sample Manager and Waters FLR Detector) with an ACCQ-TAGTM ULTRA C18 1.7 μm 2.1 × 100 mm column (Waters Corporation, USA). The excitation and emission wavelengths for fluorescent detection of amino acids were 350 nm and 450 nm, respectively. The system was operated with a flow rate of 0.25 ml/min at 30°C.
The UPLC gradient system (A = 50 mM sodium acetate (pH 5.9), B = methanol) was 10–20% B over 3.2 min, 20% B for 1 min, 20–40% B over 3.6 min, 40% B for 1.2 min, 40–60% B over 3.8 min, 60% B for 1 min, and 60–10% B over 0.01 min. Just before the analysis by UPLC, each sample (10 μl) was transferred to a UPLC tube, and NAC/OPA (20 μl) and a borate buffer (70 μl) were added; then it was left for 2 min in a dark room. The same method was used for the standard solutions containing L- and D-amino acids (aspartic acid, glutamic acid, asparagine, serine, glutamine, histidine, threonine, arginine, alanine, tyrosine, valine, methionine, tryptophan, phenylalanine, isoleucine, and leucine), glycine, taurine and γ- amino butyric acid (GABA).
Liver triacylglycerol and total cholesterol analysis
All of the lipids in the liver were extracted using the method of Folch et al. [5], with some modification. A chloroform/ methanol (1:1, vol/vol) solution (12 ml) was added to the liver sample, followed by homogenization. Chloroform (6 ml) was again added, followed by homogenization. The mixture was left for 30 min at 40°C, and filtered. A chloroform/methanol (2:1, vol/vol) solution (2 ml) and distilled water (3.6 ml) were added to the filtrate, and the mixture was stirred. The mixture was left for one day at 4°C in order to separate it into two distinct phases, and the lower phase was collected. The lower phase was dried with a nitrogen gas-evaporator, and the dried extract was diluted with 5mL isopropanol, followed by assay using triacylglycerol and cholesterol determination kits, respectively (Triglyceride E-test wako and Cholesterol E-test wako, Wako Pure Chemical Industries, Ltd., Osaka, Japan). These absorbances were analyzed by Epoch (BioTek Instruments Inc. Vermont, USA).
Statistical analysis
Data from the same treatment were analysed by one-way analysis of variance, including the initial value. When significant effects were found, the values were compared by the Tukey–Kramer test. Statistical significance was set at P < 0.05. The results are shown as means ± S.E.M. Using the data of all the mice, simple regression analysis was carried out between the liver triacylglycerol or total cholesterol contents and the plasma free amino acid concentration, after which stepwise regression analysis was also applied.
Results
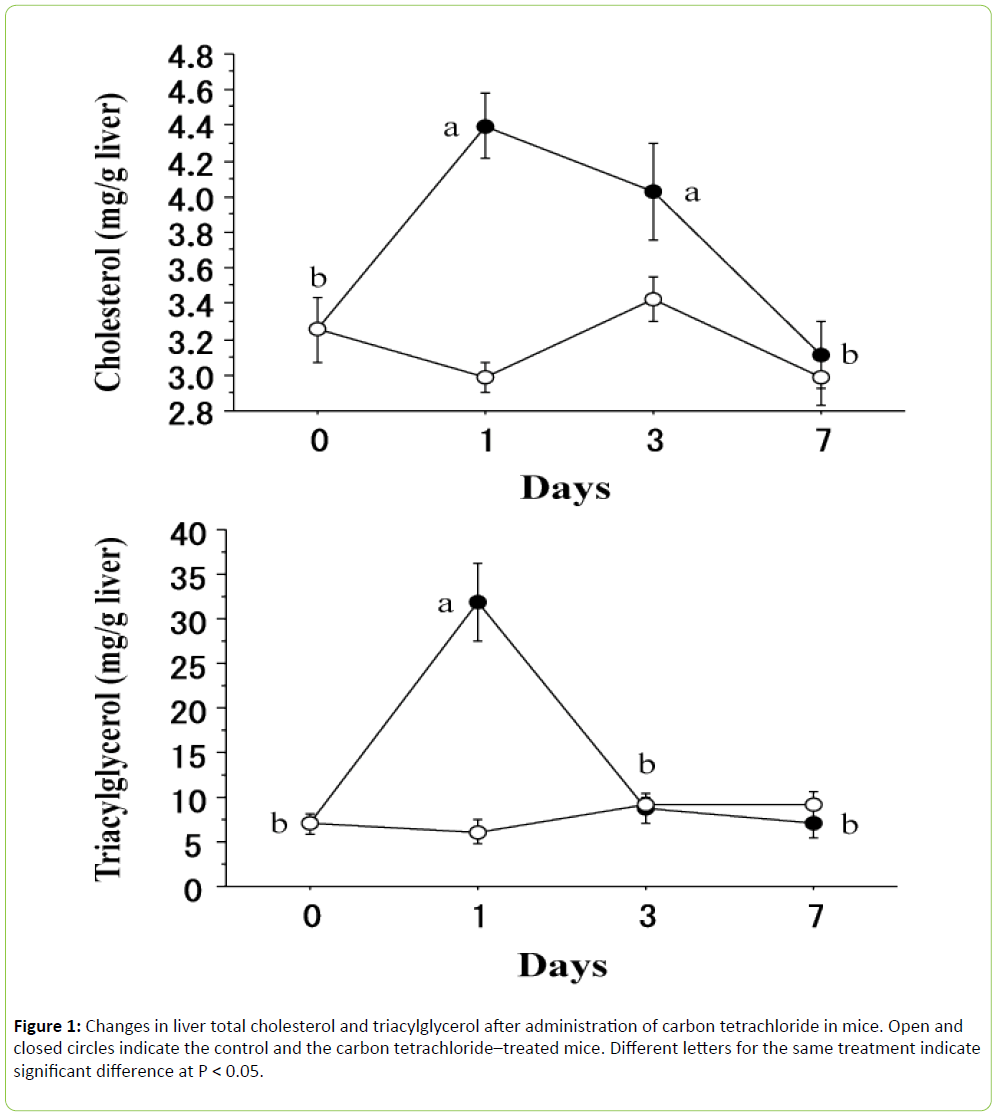
As shown in Figure 1, liver total cholesterol ((F 3, 24) = 8.681, P < 0.001) and triacylglycerol ((F 3, 24) = 21.995, P < 0.0001) contents were significantly modified by carbon tetrachloride. In the case of liver total cholesterol, the value sharply increased at 1 day after carbon tetrachloride administration and remained high for up to 3 days, and returned to the initial value at 7 days. One the other hand, liver triacylglycerol quickly increased at 1 day and returned to the initial value at 3 days.
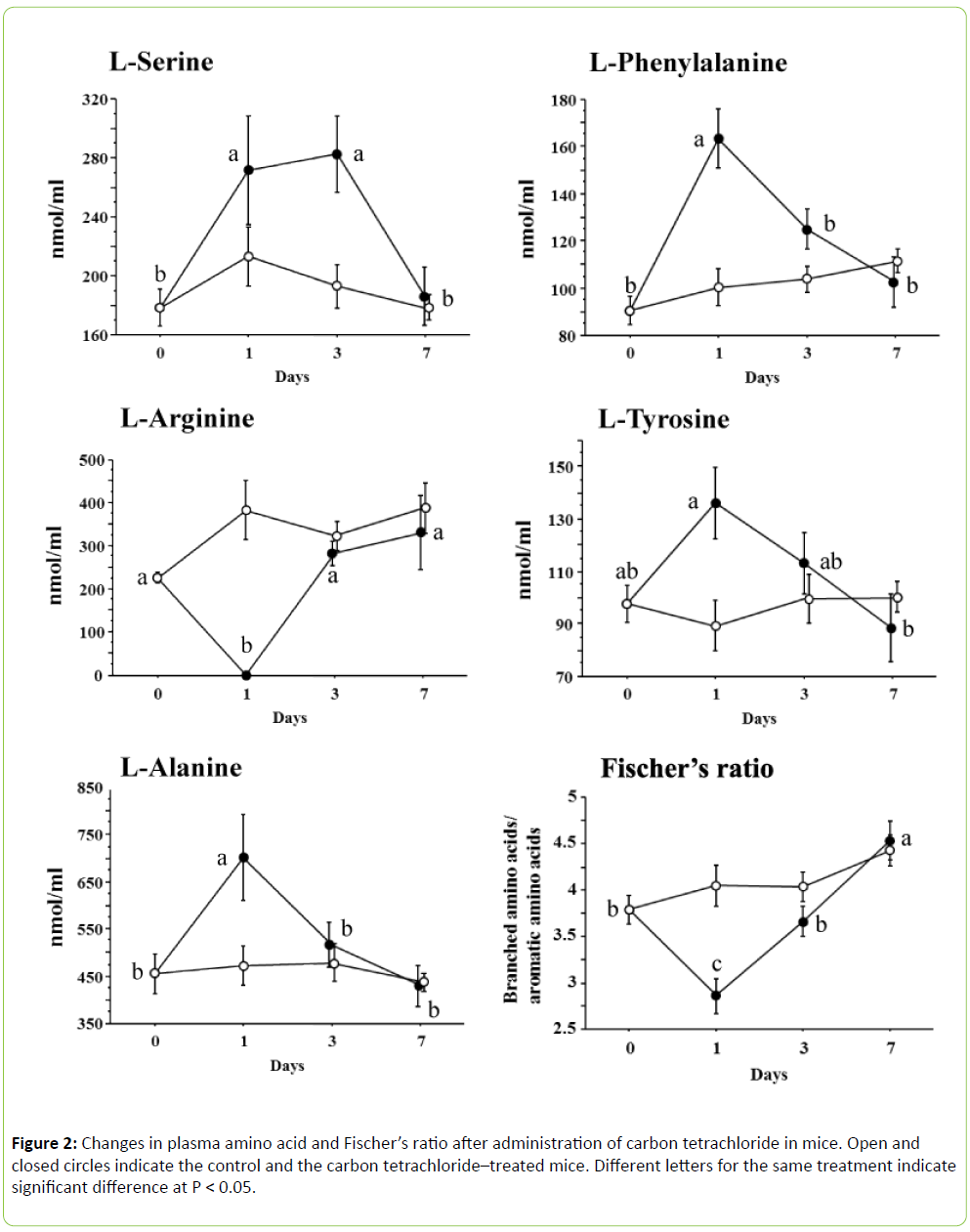
In the present study, we determined 16 amino acids of both L- and D-forms along with glycine, taurine and GABA, as mentioned above. However, only a few amino acids were significantly changed after being challenged with carbon tetrachloride (Figure 2). In the case of plasma L-serine ((F 3, 24) = 5.555, P < 0.005), the values significantly increased at 1 day and remained high for up to 3 days, but returned to the initial level at 7 days. Plasma L-phenylalanine ((F 3, 24) = 12.006, P < 0.0001), L-tyrosine ((F 3, 24) = 3.510, P < 0.05) and L-alanine ((F 3, 24) = 5.196, P < 0.01) significantly increased or tended to increase at 1 day and returned to the initial value at 3 days, but plasma L-arginine ((F 3, 24) = 10.245, P < 0.001) disappeared at 1 day and showed the initial value at 3 days. Other amino acids were not influenced by carbon tetrachloride. Fischer’s ratio significantly decreased at 1 day after administration of carbon tetrachloride and returned to the initial value at 3 days.
Significant regression equations calculated from the plasma free amino acid concentration and the liver triacylglycerol or liver total cholesterol contents are shown in Table 1. Both liver triacylglycerol and total cholesterol were positively correlated with L-alanine, L-tyrosine, L-phenylalanine and AAA, but were negatively correlated with L-arginine and Fischer’s ratio. DAlanine and L-methionine were negatively correlated with the liver triacylglycerol contents, but taurine was positively correlated. L-Aspartate and L-serine were positively correlated with the liver total cholesterol contents. The stepwise regression analysis was then applied using all the amino acids determined for liver triacylglycerol and total cholesterol as follows
| Regression equation | R2 | P |
|---|---|---|
| Triacylglycerol= | ||
| 20.617(SE3.399)–0.702(SE0.237)×D-Alanine | 0.157 | <0.005 |
| -1.692(SE4.765)+0.026(SE0.009)×L-Alanine | 0.147 | <0.01 |
| 19.995(SE2.253)–0.031(SE0.007)×L-Arginine | 0.306 | <0.0001 |
| 22.221(SE4.616)–0.097(SE0.039)×L-Methionine | 0.115 | <0.05 |
| -0.328(SE5.114)+0.113(SE0.048)×L-Tyrosine | 0.107 | <0.05 |
| -7.918(SE4.952)+0.169(SE0.042)×L-Phenylalanine | 0.256 | <0.0005 |
| 0.275(SE4.767)+0.015(SE0.006)×Taurine | 0.111 | <0.05 |
| -5.424(SE5.223)+0.077(SE0.023)×Aromaticaminoacid | 0.190 | <0.005 |
| 42.984(SE7.058)–8.093(SE1.779)×Fischer’sratio | 0.306 | <0.0001 |
| Totalcholesterol= | ||
| 2.336(SE0.304)+0.002(SE0.001)×L-Alanine | 0.239 | <0.0005 |
| 3.868(SE0.168)–0.001(SE0.001)×L-Arginine | 0.152 | <0.01 |
| 3.171(SE0.156)+0.006(SE0.003)×L-Aspartate | 0.1 | <0.05 |
| 2.190(SE0.284)+0.006(SE0.001)×L-Serine | 0.315 | <0.0001 |
| 2.290(SE0.320)+0.011(SE0.003)×L-Tyrosine | 0.234 | <0.0005 |
| 1.993(SE0.319)+0.013(SE0.003)×L-Phenylalanine | 0.324 | <0.0001 |
| 2.028(SE0.327)+0.007(SE0.001)×Aromaticaminoacid | 0.302 | <0.0001 |
| 5.767(SE0.458)–0.591(SE0.115)×Fischer’sratio | 0.358 | <0.0001 |
Table 1: Regression equations between liver triacylglycerol (mg/g liver) or liver total cholesterol (mg/g liver) and plasma amino acid concentrations (nmol/ml) in mice treated with carbon tetrachloride.
Liver triacylglycerol (mg/g liver) = 15.423 (SE 4.792) – 0.214 (SE 0.060) × L-histidine (nmol/ml) – 0.157 (SE 0.029) × Lmethionine (nmol/ml) + 0.329 (SE 0.031) × L-phenylalnine (nmol/ml), R2 = 0.753, P < 0.0001,
Liver total cholesterol (mg/g liver) = 3.929 (SE 0.550) – 0.002 (SE 0.001) × L-glutamine (nmol/ml) – 0.004 (SE 0.001) × Lvaline (nmol/ml) + 0.023 (SE 0.003) × L-phenylalnine (nmol/ ml), R2 = 0.558, P < 0.0001.
Discussion
The mechanism of carbon tetrachloride toxicity has been reviewed by Recknagel et al. [6]. The lipid accumulation caused by carbon tetrachloride is due to failure of the liver to transport triacylglycerol-rich low-density lipoproteins into the plasma. The present study confirmed rapid accumulation of both triacylglycerol and total cholesterol after administration of carbon tetrachloride. However, the response of triacylglycerol differed from that of total cholesterol, since the liver triacylglycerol level returned to the initial value at 3 days but the liver total cholesterol only did at 7 days. These responses could not be explained by the failure to transport triacylglycerol-rich low-density lipoproteins alone. Carbon tetrachloride increases the synthesis of both triacylglycerols and cholesterol in the liver [1]. The present results suggest that the stimulation of synthesis in the liver by carbon tetrachloride may take longer in cholesterol than it does in triacylglycerol.
Structural disorganization of the endoplasmic reticulum with loss of function of microsomal enzymes, including the cytoc hrome P-450 monooxygenase system and glucose-6- phosphatase, occurred coincidentally with the onset of the conditions leading to the fatty liver [6]. This loss of microsomal enzyme function may be due to the inhibition of enzyme synthesis, since protein synthesis was rapidly retarded by carbon tetrachloride [7]. Retarded protein synthesis influences the plasma free amino acid pool. In particular, this phenomenon is characterized by a decrease in BCAAs and an increase in AAAs: the BCAAs are catabolized by both fat and muscle and the AAAs cannot be catabolized by the damaged liver [8]. In the present study, AAAs in the form of Lphenylalanine and L-tyrosine were significantly increased, but no BCAA was modified after administration of carbon tetrachloride. We calculated Fischer’s ratio, which was significantly decreased at 1 day. However, this was due to the increase in AAAs without a reduction in BCAAs. Holecek et al. [9] compared plasma free amino acids of rats with carbon tetrachloride–induced acute liver damage and carbon tetrachloride–induced liver cirrhosis. In the case of acute liver damage, they reported extreme elevation of most amino acids, including BCAAs, a profound decrease of arginine and an unchanged Fischer’s ratio. On the other hand, the liver cirrhosis increased the levels of AAA, methionine, ornithine, asparagine, and aspartate, and decreased the BCAA concentrations. As a result, Fischer’s ratio decreased significantly. Muratsubaki and Yamaki [4] reported that carbon tetrachloride induced acute liver damage in rats, with all amino acids (except for arginine, which disappeared) significantly increasing and with Fischer’s ratio significantly decreasing. We used mice in the current study, which differs from previous reports on rats. Thus, differences in species, doses of carbon tetrachloride, severity of liver damage, nutrition and so on may explain the different responses found among experiments. In the present study, L-alanine and Lserine specifically increased with administration of carbon tetrachloride. Both amino acids are gluconeogenic amino acids and L-alanine has a key role in this process. The kidneys, gut and muscle provide alanine, and the kidneys also provide a major source of serine for uptake by the liver [10]. However, carbon tetrachloride inhibited liver gluconeogenesis [11]. As a result, plasma L-alanine and L-serine levels increased.
We tried to clarify the relationships between liver lipid levels and single plasma free amino acid concentration. Plasma L-alanine, L-tyrosine, L-phenylalanine and AAA reflected and positively correlated with both liver triacylglycerol and total cholesterol. However, the R2 value was low in each amino acid (Table 1). Thus, the stepwise regression analysis was applied using all amino acids determined for liver triacylglycerol and total cholesterol. In the cases of liver triacylglycerol and total cholesterol, both equations contained L-phenylalanine, but not L-tyrosine. These results imply that the increase in plasma free L-phenylalanine plays a key role in the accumulation of liver lipid.
In conclusion, the liver lipid contents could be estimated using the plasma free amino acid concentration following the administration of carbon tetrachloride. In case of acute liver damage induced by the administration of carbon tetrachloride, plasma free L-phenylalanine was an important factor for accumulation of both triacylglycerol and cholesterol in mice.
References
- Weber LWD, Boll M, Stampfl A (2003) Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Critical Reviews in Toxicology 33: 105-136.
- Holecek M (2015) Ammonia and amino acid profiles in liver cirrhosis: effects of variables leading to hepatic encephalopathy. Nutrition 31: 14-20.
- Fischer JE, Rosen HM, Ebeid AM, James JH, Keane JM, et al. (1976) The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery 80: 77-91.
- Muratsubaki H, Yamaki A (2011) Profile of plasma amino Acid levels in rats exposed to acute hypoxic hypoxia. Indian Journal of Clinical Biochemistry 26: 416-419.
- Folch J, Lees M, Slone Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry 226: 497-509.
- Recknagel RO, Glende EA Jr, Dolak JA, Waller RL (1989) Mechanisms of carbon tetrachloride toxicity. Pharmacology & Therapeutics. 43: 139-154.
- Smuckler EA, Iseri OA, Benditt EP (1962) An intracellular defect in protein synthesis induced by carbon tetrachloride. Journal of Experimental Medicine 116: 55-72.
- Soeters PB, Fischer JE (1976) Insulin, glucagon, aminoacid imbalance, and hepatic encephalopathy. Lancet 2: 880-882.
- Holecek M, Mraz J, Tilser I (1996) Plasma amino acids in four models of experimental liver injury in rats. Amino Acids 10: 229-241.
- Rodwell VW (2009) Catabolism of proteins & of amino acid nitrogen. In: Harper's Illustrated Biochemistry, (28thedn) McGraw Hill Professional, New York, pp: 239-247.
- Faus MJ, Lupiáñez A, Vargas A, Sánchez-Medina F (1978) Induction of rat kidney gluconeogenesis during acute liver intoxication by carbon tetrachloride. Biochemical Journal 174:461-467.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences