Effect of Vitamin C and D on Growth and Immunology of Rohu La ( beo Rohita) Fingerlings in District Lahore
Aamira Urooj, Muhammad Ashraf, Aasma Nourin, Masroor Elahi Babar, M Tariq Pervaiz, and Tanveer
Published Date: 2022-04-12DOIDOI: 10.36648/2572-5459/7.4.16
Aamira Urooj*, Muhammad Ashraf, Aasma Nourin, Masroor Elahi Babar, M Tariq Pervaiz, and Tanveer
Department of Science and Technology, Virtual University, Pakistan
- *Corresponding Author:
- Aamira Urooj, Department of Science and Technology, Virtual University, Pakistan; Tel: 03074469162; E-mail: m.ashraf@vu.edu.pk
Received: 14-Mar-2022, Manuscript No. IPJARN-22-12223; Editor assigned: 17-Mar-2022, Pre QC No. IPJARN-22-12223 (PQ); Reviewed: 31-Mar-2022, QC No. IPJARN-22-12223; Revised: 5-Apr-2022, Manuscript No. IPJARN-22-12223 (R); Published: 12-Apr-2022, DOI: 10.36648/2572-5459/22.7.11
Citation: Urooj A, Ashraf M, Nourin A, Elahi Babar M, Pervaiz MT, et al. (2022) Effect of Vitamin C and D on Growth and Immunology of Rohu (Labeo Rohita)Fingerlings in District Lahore. J Anim Res Nutr Vol:7 No:2
Abstract
The present study was scheduled for 90 days to determine the effect of vitamin C and D on growth and immunology of Rohu (Labeo rohita) fingerlings at Fisheries Research and Training Institute, Manawan district Lahore, Punjab, Pakistan in 12 different ponds (July 1 to September 30, 2019) with 3 different treatments and a control one. Four levels of treatment were used vitamin C (0.00, 0.23 g, 0.48 g, and 0.69 g) and vitamin D (0.00, 0.26 g, 0.43 g, and0.68 g) in triplicate groups. Supplementary feed that was composed of sunflower meal, maize gluten meal and rice polish (1:1:1) was applied to fish @ 2% of fish body weight twice a day. Artificial diets were formulated which contained 32.20% crude protein. All the water quality assessments/parameters fluctuated in values during the course of an experiment like temperature â??, dissolved oxygen, pH, carbon dioxide, alkalinity, total hardness and planktonic biomass. All these parameters were tested on every 10th day so each pond was sampled thrice in a month. Final weight was (wt) 265.4 ± 4.15 g (control), 272.5 ± 2.42 g (T1), 280.5 ± 2.80 g (T2) and 291.0 ± 5.75 g (T3) respectively. SGRs were 0.6 ± 0.01% (control), 0.6 ± 0.01% (T1), 0.8 ± 0.01% (T2) and 0.5 ± 0.03% (T3) respectively. Weight gain was 18.6 ± 0.75 g (control), 20.0 ± 0.47 g (T1), 24.8 ± 2.25 g (T2) and 23.4 ± 1.68 g (T3) respectively. Survival rate (%) was 56.6 ± 2.51% (control), 60.3 ± 3.51% (T1), 85.3 ±2.51% (T2) and 72.3 ± 1.52% (T3) respectively. Average daily weight gain was (ADG) 0.2 ± 0.01 (control), 0.2 ± 0.01 (T1), 0.2 ± 0.01 (T2) and 0.2 ± 0.01 (T3) respectively which showed better results than other treatment groups. The vitamin C (AAs) and vitamin D was resolved in blood erythrocyte (1.5 ± 0.06 (T1), 1.5 ± 0.08 (T2), 2.3 ± 0.06 (T3) and 1.8 ± 0.12 (T4) respectively, hemoglobin 4.6 ± 0.30 (T1), 5.9 ± 0.73 (T2), 7.8 ± 0.25 (T3) and 7.1 ± 0.05 (T4) respectively). Body flesh enhanced the growth and immune system of fish. In control group (without vitamin C and D diet) hemoglobin levels decreased which might have decreased the oxygen carrying capacity in fish. Increased bacterial infection was perceived in the group which was fed on supplemented diet without vitamin C and D. Supplementary diet T2 indicated significantly (p<0.05) higher growth than the other treatments and control one. In Pakistan, supplementary diets of vitamin C and D were affected on the immunological parameters and growth assessment of fish Rohu (Labeo rohita).
Keywords
Hematology; Immunological parameters; Growth performance; Water Quality parameters; Average daily weight gain; Survival rate; Mortality rate; Hemoglobin; Erythrocytes; Ascorbic acid (AA); Immunomodulation
Introduction
Fish body cannot synthesize vitamin C and D endogenously rather they have to depend on the exogenous sources provided by the artificial food. Fish can store huge amount of vitamin D in their fat tissue, liver and fat muscles if supplied from outside sources. In natural condition, planktonic biomass is rich source of vitamin C and D. In artificial ponds/tanks vitamin D are provided by the supplementation of different formulated diets [1].
Vitamin C and D are playing a very vital role in many nutritional and physiological functions. The vitamin C is easily soluble in water and vitamin D is soluble in fat. Vitamin D is essential to maintain the phosphate and Calcium and to protect the skeleton and maintaining the bone mineralization [2]. Due to non-vitaminC supplementation diets fish showed pathological changes like weakness of spinal bones, vertebral curvature and retarded growth [3]. Fish responded by increased or decreased level of ascorbate in the availability of diets. The dietary concentrated vitamin C and D were equal to the perpetuation of “steady-state” enhancement of tissue in fingerling and larval stage of fish [4]. In fish farms vitamins C and D were used for the bacterial defense mechanism in fish [5].
During tissue formation ascorbic acid enriched feed is provided to fingerlings stage for proper growth and survival. Zooplankton (Diaptomus, Daphnia, Cyclops, Moina) are rich source of vitamin C (ascorbic acid/ascorbyl palmitate) [6]. Dietary vitamin D and C were necessary for fish because it depends on different factors for survival and growth like environmental/ecological, developmental, genetic conditions and physiological modes [2]. The growth and immunomodulation of Rohu (Labeo rohita) showed the positive results in the presence of carnitine [7].
Fish cannot synthesize cholecalciferol and Ascorbic Acid (AA) for their growth. Hematological parameters, growth parameters, serological parameters and immunological parameters were increased by high level of vitamin C supplementary diet [8]. Fish body flesh or structure obtained huge quantities of vitamin D (cholecalciferol) by feeding the diets from external source. Near the surface of water, the planktonic biomasses act as a pro-vitamin D source. In planktonic biomass vitamin D (cholecalciferol) was synthesized by the exposure of solar VU light on the water body [9]. In fish vitamin D is synthesized by support of endocrine system as in mammals. Vitamin D activates the plasma level in blood of fish. Protein‐ Bound Transport (PBT) in blood plasma is more active in vitamin D. Vitamin D acts as receptor and response distribution to the target tissues like bone, kidney, gills and intestine [1].
Specific Growth Rate (SGR) and Food Conversion Ratio (FCR) are partly based on the availability of the supplemented dietary vitamin D3 as in the form of rotifer and algae. Muscle crude protein totally depends on the vitamin D3 and the estimated requirements of crude protein (4.85 I.U./g to 5.53 I.U./g) [10]. The supplementary diet of vitamin C (fruits of lapsi) enhanced the growth, specific growth rate, efficiency of brain and liver tissues in C. carpio [11]. If the vitamin C (ascorbic acid) was increased up to the level of requirement in fish diet, then the concentration of hepatic ascorbic acid automatically increased and vice versa [12].
Material and Methods
Study area and experimental designDuring the study period, stocking of fish species, feeding, growth performance, and water quality analysis were carefully undertaken. The water depth and average area of ponds were 2 m and 0.005 ha respectively. 500 rohu (Labeo rohita) fingerlings were stocked in each pond of 0.005 ha.
Pellet formation: All the ingredients sunflower meal, maize gluten meal and rice polish (1:1:1) were thoroughly ground and pulverized into powder. All the diets contained 32.20% protein and then in each diet was added the calculated value of vitamin C and D. These constituents were mixed well according to their formula and then dumped into the machine for the formation of pellet size of 1 mm. The composition of pellet feed is shown in Table 1.
Table 1: Percent ingredient contribution in each formulated diet.
| Ingredients | control (g/kg diet) | T1 (g/kg diet) | T2 (g/kg diet) | T3 (g/kg diet) |
|---|---|---|---|---|
| Sunflower meal (%) | 30 | 30 | 30 | 30 |
| Maize gluten meal (%) | 30 | 30 | 30 | 30 |
| Rice polish (%) | 20 | 19.51 | 19.09 | 18.63 |
| Vitamin C (AA) | 20 | 20 | 20 | 20 |
| Vitamin D (folic acid) | 0 | 0.26 | 0.43 | 0.68 |
| Poultry byproduct meal | 20 | 20 | 20 | 20 |
| Crude protein % | 32.2 | 32.2 | 32.2 | 32.2 |
Feeding and fertilization in ponds: Four experimental dietary feeds were formulated to contain different levels of vitamin C (0.00, 0.23, 0.48, 0.69 g/kg diet) and D (0.00, 0.26, 0.43, 0.68 g/kg diet) and supplementary feed that was composed of other ingredients (sunflower meal, maize gluten meal, rice polish and poultry by product meal) with the same level in four treatments (30,30,20,20 g/kg diet) respectively. The fish was fed @ 2% of fish body weight and feed was adapted according to increases in their body weight. The percentage of feed was increased by proportionate increase in their body weight. All the groups were fed daily twice @ 2% of their wet biomass at 7 am to 8 am and 4 pm to 5 pm.
Fish sampling and growth assessment: Fish was sampled three times in a month (after 10 days) with initial reading at the start of the trial for 3 months. The fish present in the pond was weighed and measured at each sampling while at the time of final sampling fish samples were collected for growth and immunological parameters too. During this whole trial all the inputs applied to the ponds were regularly recorded to find out growth-input-water quality interactions. To assess the fish growth, the final weight (g), growth rate (SGR% bwd-1), the Weight Gain (g), Average Daily Gain (ADG) in (g), and survival rate (%) of fish were sampled by seine net three times in a month. Growth assessment parameters were calculated by [13].
Final weight (g)=Weight of fish at harvest
Weight gain (g)=Mean final weight-Mean initial weight (g)
SGR (% bwd-1)=(ln final weight-ln initial weight)/Culture period × 100
ADG (g)=(Final weight-Initial weight)/culture period
Survival rate (%)=[(No. of fish harvested/No. of fish stocked)] × 100
Erythrocyte: Erythrocytes were measured by the blood sample taken from the caudal vein by a sterile syringe which contained EDTA as a chelating or coagulating agent. Blood was used to measure the erythrocyte count and hemoglobin content. Erythrocytes were calculated by plasma which was centrifuged at 3000 rpm for 15 min and then non-hemolyzed plasma was stored at 20℃ in freezer [13].
Hemoglobin: Hemoglobin was measured by the blood sample taken from the vein by a sterile syringe with 1 cc lithium oxalate to prevent the clotting of the blood. This oxalated blood was poured into the volumetric flask and then added 2 cc iron-free concentrated sulfuric acid in it. Then blood was agitated by adding the potassium sulphate 20 cc for just 10 min. Diluted with 20 cc of distilled water and then added 2 cc of 10% sodium tungstate into the flask after shaking well then cooled at room temperature. After making the solution, to dilute it up to 50 cc volume this was filtered by dry filter paper. After this took 50 cc filtered solution into the test tube and then added into it 1 cc standard ferrous ammonium sulphate containing 0.1 mg of Fe per cc. Concentrated sulfuric acid (0.8 cc) was added and then diluted up to 20 cc. The solution was cooled at room temperature. After cooling both blood solutions 1 cc of saturated potassium per-sulphate was added. Potassium sulfocyanate (0.4 cc of 3 N) was also added which contained (40 cc) acetone.
By mixing the several reagents into the test tube reading was read against unknown solution (standard solution) on colorimeter. The sample of blood contained small amount of iron standard 0.05 mg of Fe. Then Fe per 100 cc of blood was calculated by [14].
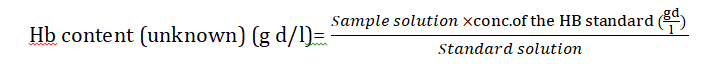
White blood cells: WBCs were measured by the blood sample taken from the caudal vein by a sterile syringe which contained EDTA as a chelating or coagulating agent. WBCs were calculated by Neubaur heamocytometer. Blood were diluted 1:20 and then placed in to the haemocytometer. Four corners into the heamocytometer were counted into microscope. Total no. of WBCs calculated in mm3 × 103.
Total WBCs (mm-3)=No. of leucocytes in 4 corners of 1 mm2
4 × 1/10 × 1/200
=Total no. of cells × 50 cells mm-3
Monitoring of physico-chemical parameter: Plastic bottles with double stoppers having a volume of 500 ml of each and marked with ponds number, were used for collection of water samples. The water quality assessments to be monitored were temperature, dissolved oxygen, pH, carbon dioxide, alkalinity and planktonic biomass [15]. The values of DO, temperature and pH were recorded at three different depths means surface, column and bottom. Samples along with different parameters were also monitored to assess water composition and plankton productivity. All the water quality parameters were tested on every 10th day so each pond was sampled thrice in a month.
Statistical analysis: For statistical analysis of the data, ANOVA was applied to compare means of different treatments [16]. Differences among treatments were distinguished by Duncan’s Multiple Range Test. Probability level was fixed at p<0.05. SPSS statistical package was used for all statistical work.
Results
Water quality parameters: In the present study, water quality parameters have great impact on the maintenance of production of food and healthy environment for the harvested fishes. Very reliable factors of water quality parameters (Table 2) were tried to maintain during the course of fingerling culture. The mean values of temperature recorded during the whole study of experiment were (control group) 19.9 ± 0.20 (⁰C), (T1) 20.6 ± 0.52 (⁰C), (T1) 20.9 ± 1.7 (⁰C) and (T3) 22.7 ± 0.87 (⁰C) respectively. The mean value of pH was recorded from four ponds in this present study in four different graded levels 7.4 ± 0.26 (control group), 7.7 ± 0.20 (T1), 8.7 ± 0.75 (T2) and 8.9 ± 0.40 9 (T3) respectively. The mean values of carbon dioxide measured in the present study under different graded levels were 121.0 ± 0.36 (mgL-1) (control group), 125.7 ± 3.66 (mgL-1) (T1), 143.7 ± 5.25 (mgL-1) (T2) and 159.4 ± 6.92 (mgL-1) (T3) respectively. The average values of dissolved oxygen (DO) evaluated in the whole study time in the four treatments were 8.3 ± 0.15 (mgL-1) (control group), 8.4 ± 0.20 (mgL-1) (T1), 8.6 ± 0.47 (mgL-1) (T2) and 9.6 ± 0.25 (mgL-1) (T3) respectively. The average values of alkalinity measured in this present study under these treatments were 142.3 ± 0.20 (mgL-1) (control group), 145.4 ± 3.02 (mgL-1) (T1), 150.8 ± 4.26 (mgL-1) (T2) and 161.2 ± 3.51 (mgL-1) (T3) respectively. The average value of calcium measured from four different graded levels ranged 130.2 ± 0.75 (mgL-1) (control group), 131.5 ± 0.36 (mgL-1) (T1), 132.7 ± 1.78 (mgL-1) (T2) and 134.9 ± 0.77 (mgL-1) (T3) respectively. The average values of planktonic biomass in the present study of ponds were 137.7 ± 1.01 (mgL-1) (control group), 138.4 ± 1.37 (mgL-1) (T1), 147.5 ± 8.1 (mgL-1) (T2) and 158.4 ± 0.95 (mgL-1) (T3) respectively.
Table 2: Variations are shown in the mean values of water quality parameters under different treatments during the study period.
| Parameters | Control | T1 | T2 | T3 |
|---|---|---|---|---|
| Temperature (⁰C) | 19.9 ± 0.20 a | 20.6 ± 0.52 a | 20.9 ±1.7 a | 22.7 ± 0.87 a |
| pH | 7.4 ± 0.26 b | 7.7 ± 0.20 b | 8.7 ±0.75 a | 8.9 ± 0.40 a |
| CO2 (mgL-1) | 121.0 ± 0.36 c | 125.7 ± 3.66 b | 143.7 ± 5.25 a | 159.4 ± 6.92 a |
| Alkalinity (mgL-1) | 142.3 ± 0.20 a | 145.4 ± 3.02 a | 150.8 ± 4.26 a | 161.2 ± 3.51 a |
| DO (mgL-1) | 8.3 ± 0.15 b | 8.4 ± 0.20 b | 8.6 ± 0.47 b | 9.6 ± 0.25 a |
| Ca (mgL-1) | 130.2 ± 0.75 c | 131.5 ± 0.36 b | 132.7 ± 1.78 a | 134.9 ± 0.77 a |
| T.S (mgL-1) | 976.6 ± 9.0 d | 1032.6 ± 10.5 c | 1057.6 ± 11.6 b | 1080.3 ± 12.6 a |
| Planktonic biomass (mgL-1) | 137.7 ± 1.01 d | 138.4 ± 1.37 c | 147.5 ± 8.1 b | 158.4 ± 0.95 a |
Means with the same superscript letters in the same row were not significantly different (p<0.05). Data are presented as mean ± SD;
Growth assessments: The mean values of initial weight (g) measured from different graded experiments in present study were 215.4 ± 2.09 g (control group), 217.8 ± 2.32 g (T1), 220.8 ± 3.26 g (T2) and 226.0 ± 4.6 g (T3) respectively. The average values of final weight (g) observed from different graded levels was 265.4 ± 4.15 g (control group), 272.5 ± 2.42 g (T1), 280.5 ± 2.80 g (T2) and 291.0 ± 5.75 g (T3) respectively. Mean values of weight gain (%) measured from the four different graded treatments in present study were 18.6 ± 0.75 (%) (Control group), 20.0 ± 0.47 (%) (T1), 24.8 ± 2.25 (%) (T2) and 23.4 ± 1.68 (%) (T3) respectively. The mean values of average daily gain (%) calculated in the whole study time were 0.6 ± 0.01 (%) (Control group), 0.6 ± 0.01 (%) (T1), 0.8 ± 0.01 (%) (T2) and 0.5 ± 0.03 (%) (T3) respectively. The mean values of specific growth rate measured from the different levels 0.2 ± 0.01 (control), 0.2 ± 0.01 (T1), 0.2 ± 0.01 (T2) and 0.2 ± 0.01 (T3) respectively. The mean values of survival rate (%) observed in four different treatments observed in pond of Rohu carp (Labeo rohita) were 56.6 ± 2.51 (%) (Control group), 60.3 ±3.51 (%) (T1), 85.3 ± 2.51 (%) (T2) and 72.3 ± 1.52 (%) (T3) respectively. The mean value of mortality rate (%) measured in the present study was 43.3 ± 2.51 (%) (Control group), 39.6 ± 3.15 (%) (T1), 14.6 ± 2.51 (%) (T2) and 27.6 ± 1.52 (%) (T3) respectively. These average values of erythrocyte examined in the present study were 1.5 ± 0.06 (c/mm3 control group), 1.5 ± 0.08 (c/mm3 T1), 2.3 ± 0.06 (c/mm3 T2) and 1.8 ± 0.12 (c/mm3 T3) respectively.
Mean values of hemoglobin observed were 4.6 ± 0.30 (g/100 ml control group), 5.9 ± 0.73 (g/100 ml T1), 7.8 ± 0.25 (g/100 ml T2) and 7.1 ± 0.05 (g/100 ml T3) respectively. These average values of white blood cells examined in the present study were 5.0 ± 0.04 (mm3 × 103 control group), 5.9 ± 0.48 (mm3 × 103 T1), 6.9 ± 0.05 (mm3 × 103 T2) and 7.1 ± 0.03 (mm3 × 103 T3) respectively Table 3.
Table 3: Effect of Vitamin C and D on growth and concentrations of erythrocytes and hemoglobin in Rohu (Labeo rohita) fingerlings fed on experimental diets for 90 days.
| Parameters | Control | T1 | T2 | T3 |
|---|---|---|---|---|
| Final weight (g) | 265.4 ± 4.15 d | 272.5 ± 2.42 c | 291.0 ± 5.75 a | 280.5 ± 2.80 b |
| Weight gain % | 18.6 ± 0.75 c | 20.0 ± 0.47 b | 24.8 ± 2.25 a | 23.4 ± 1.68 a |
| ADG (g) | 0.6 ± 0.01 c | 0.6 ± 0.01 c | 0.8 ± 0.01 a | 0.5 ± 0.03 b |
| SGR (% bwd-1) | 0.2 ± 0.01 a | 0.2 ± 0.01 a | 0.2 ± 0.00 a | 0.2 ± 0.00 a |
| Survival rate (%) | 56.6 ± 2.51 d | 60.3 ± 3.51 c | 85.3 ± 2.51 a | 72.3 ± 1.52 b |
| Mortality rate (%) | 43.3 ± 2.51 a | 39.6 ± 3.15 b | 14.6 ± 2.51 c | 14.6 ± 2.51 c |
| Erythrocyte (c/mm3) | 1.5 ± 0.06 c | 1.5 ± 0.08 c | 2.3 ± 0.06 a | 1.8 ± 0.12 b |
| Hemoglobin (g/100 ml) | 4.6 ± 0.30 d | 5.9 ± 0.73 c | 7.8 ± 0.25 a | 7.1 ± 0.05 b |
| WBCs (mm-3) | 5.0 ± 0.04 d | 5.9 ± 0.48 c | 6.9 ± 0.05 b | 7.1 ± 0.03 a |
Means with the same superscripts letters in the same row were not significantly different (p<0.05). Data are presented as mean ± SD;
Discussion
During the study period, the fish (Labeo rohita) fingerlings were fed on supplementary diets containing different levels of vitamin C and D for the period of 90 days. Fish growth, Specific growth rate, and average daily gain of fish are conducted by the change of environmental elements [17]. In the present study, 500 rohu (Labeo rohita) fingerlings were stocked, water depth and area of the ponds was 2 m and 0.005 ha respectively are agreed with previous study as used neither shallower nor deep ponds more than 5 m [18].
In this study, the mean values of temperature recorded during the whole study of experiment were shown minor fluctuation but the temperature 22⁰C (T3) was higher than the other two treatments and control one. This measured value was suitable as an experiment on temperature fluctuations (20-26°C) and its effects on fish for the period of ninety days [19]. The experimental values of previous study as studied that the water temperature ranged from 20.50⁰C to 36.50⁰C in pond water [20]. Scientist observed the fluctuation in temperature from 26.5 to 32.5°C [21]. In 2007 also observed the mean value of temperature 30.9-31.1⁰C [22].
The mean value of pH was recorded from four ponds in this present study, the control group diet and T1 are shown the less significance than the T2 and T3. This study was more similar to the results [23,24]. In water quality assessments, the average values of CO2 did not show significant difference in these four treatments of present study. In T3 and T2 showed the greater significance. Our values of CO2 are much agreement with scientist observed in their study that CO2 mean value ranged from 210 mg L-1 to 278 mg/L [25].
The value of DO in T3 is higher than the other treatments with minimum fluctuations. This study agrees that dissolved oxygen in different levels which ranged from 2.0-7.4 mg/L [26]. This is very similar to the finding of scientist who observed the DO from ponds in the range of 3.4-8.97 mg/L [27]. In the present study, the mean value of alkalinity did not show the significance difference in four treatments. But these values have shown little bit fluctuation. Like previous study, the alkalinity of ponds which ranged from 96-127 mg/L [28]. Scientist also found the total alkalinity was more than 20 mg/L [25]. The mean value of calcium was recorded from four ponds in this present study, the control group diet, T1 and T2 showed less significance than the T3. The concentration of the calcium content remained between 143.11 mg L-1 to 471.21 mg per L [29]. This is very similar to the findings who noticed the mean values of planktonic biomass which fluctuated from 130 mg per L to 185 mg per L [29].
In the present study, the initial weight of these four treatments is almost same. And the values of initial weight are very much similar to the previous studies according to the initial weight was 3.5 ± 0.1 g in their study of treatment T1 [30], and these findings are quite similar to the initial weight of fishes in their experiment which was 9.4 ± 0.58[31]. These findings are lower than the present study because the initial weight of fingerlings was much high at the time of stocking so used the fingerlings with initial body weight of 1.42 ± 0.25 g [32]. The final weight of larval and juvenile fish tissue increased from 76 to 113 mg with vitamin C supplementation in fish4. The initial body weight of 35 ± 5 g of fish in their trials [33].
The main value of average daily gain (ADG) was shown that the difference between T1 is higher than the T2. As in previous study conducted the feed regulation and loss in weight gain was affected by hypervitaminosis from D or vitamin D [34]. This study is quite similar to scientist who reported that the ascorbic acid showed better production rate and significant difference in the daily weight gain (WG) of parrot fish (Scaridae) [35]. The present study had disagreed with observed weight gain (%) (21.7 to 16.8 g per fish) in their 12 weeks of trial on Heterobranchus longifilis fingerlings [3].
The concentrations of vitamin C in T2 and T3 enhanced the survival rate as observed increased specific growth rate (%), survival (%) and the body weight gain (g) (p<0.05) with the inulin and vitamin C supplementation after feeding of 2 months fed diet [36]. As the above-mentioned values in the affected range which was quite similar to study the growth rate, survival rate and maximum growth performance based on the dietary requirement of 45 mg kg-1 ascorbic acid [37]. This agrees well with the finding of during the experiment that the vitamin C as supplemented polyphosphorylated L-ascorbic acid (200 and 400 C2PP mg kg-1) and showed the higher survival rate (%) [38]. The survival rate increased then the rate of production also increased as previous study like measured the large value of Growth performance, survival rate and body weight gain by fish fed the high dosage of vitamin C in mrigal (Cirrhinus mrigala) fingerlings [39].
Mortality rate increased with the non-vitamin C diet which supported the observations of scientist who measured the quantitative dietary AA requirements of fingerling Nile tilapia [40]. Non-ascorbic acid diets showed the deficiency symptoms like mortality, anorexia. Present studies collaborate with that fed fish on less ascorbic acid diet which in turn caused the deficiency such as anorexia, high mortality, and retarded growth and darkening of body color and liver showing the symptom of atrophy [35]. According to the finding of experiments on salmon and trout and showed higher rate of mortality after feeding on diet without vitamin C supplementary diet [41].
Due to high dose of vitamin C and D (T2 and T3) more leucocytes (WBCs) were produced which is similar to the study who reported that on the low level of vitamin C which showed delay or inhibited wound repair until fish dies [41]. This present study was depicted to the WBCs are calculated when fish were treated by the vitamin C and D [42]. Increased bacterial infection was perceived in the group which was fed on supplemented diet without vitamin C and D. As the production of WBCs was the sign of protection of harmful bacteria as well as the higher concentrations of vitamin C showed high resistance to bacterial infections in the blood serum [39]. In the previous studies the respiratory burst activity (oxidative burst activity) of blood erythrocyte and neutrophils which significantly increased with higher concentration of vitamin C (Ascorbyl polyphosphate of 100 mg per kg diet) in fish [43].
The high content of hemoglobin was indicating the fish health (quality and quantity) agrees well with finding of high supplementation of vitamin C (Ascorbic Acid) which increased the hematological parameters, and immune-modulation system [8]. These findings collaborate with previous study without vitamin C diets there was decrease in the hemoglobin and hematocrit levels in the blood of fish [3]. The decreased free radical indicated also decreased in the hemoglobin in blood which also agrees with who studied ascorbic acid which increased the metabolism of trout for conception of iron in their blood [44]. And the vitamin C (ascorbic acid) increased the efficiency of absorption of iron in intestinal track. The higher concentration of vitamin C (1000 mg per kg) increased the hemoglobin content in blood [45]. Same results were shown in previous study who measured the better results showed in the treatment of fish fed with 210 mg AA per kg [33]. This agrees well with the findings who observed that with the decrease in the concentration of vitamin C in their diet there was proportionate decrease in blood levels of vitamin C [41]. The higher concentration of vitamin C increased the hemoglobin and hematocrit in mrigal (Cirrhinus mrigala) fingerlings [39].
Higher doses of vitamin C and D (T2 and T3) produced the erythrocytes (RBCs) which agree that they supplemented the dietary vitamin C @ 400 (milligram ascorbic acid per kilogram diet) and this level of supplementation showed increased erythrocyte and hemoglobin (Hb)[46]. In the previous study, the hematocrit and erythrocyte from the lysozyme activity in the blood serum [36]. Non-vitamin C and D supplementary diet indicated that the performed an experiment and observed that when fish were fed on just basal diets, fish blood showed lower levels of red blood cells as well as hemoglobin levels than those fishes which were offered after enriching them with vitamin C [47]. When fish feed containing 500 (mg kg-1) of vitamin C was offered to fish per month, it directly affected hematology, growth, resistance to diseases in Nile tilapia (O. niloticus) and innate immunity [36].
Acknowledgements
God never spoils any effort. I am thankful to ALMIGHTY ALLAH and HOLY PROPHET MUHAMMAD (PBUH) who is the most benefactors in my all life works and enable me to overcome difficulties in my scientific work. I am very glad to express my sincere thanks to my respected Supervisor Prof. Dr. Muhammad Ashraf, for his expert suggestions, consistent encouragement, special attention, and constructive criticism. I also acknowledge to my respected teacher Dr. Aasma Noureen who despite their busiest academic schedules acted as spiritual teachers. I also acknowledge to my beloved parent, siblings for his financial support, kind help, spiritual support, moral support, sacrifices.
References
- Lock E J, Waagbø R, Wendelaar Bonga S, Flik G (2010) The significance of vitamin D for fish: a review. Aquac Nutr 16:100-116
- Darias M J, Mazurais D, Koumoundouros G, Cahu C L, Zambonino-Infante JL, et al. (2011) Overview of vitamin D and C requirements in fish and their influence on the skeletal system. Aquac 315:49-60
- Ibiyo LM, Madu CT, Eze SS (2006) Effects of vitamin C supplementation on the growth of Heterobranchus longifilis fingerlings. Arch Anim Nutr 60:325-332
- Dabrowski K (1990) Ascorbic acid status in the early life of whitefish (Coregonus lavaretus L.). Aquacu 84:61-70
- Montero D, Marrero M, Izquierdo MS, Robaina L, Vergara JM, et al. (1999) Effect of vitamin E and C dietary supplementation on some immune parameters of gilthead seabream (Sparus aurata) juveniles subjected to crowding stress. Aquacu 171:269-278
- Mitra G, Mukhopadhyay PK (2003) Dietary essentiality of ascorbic acid in rohu larvae: Quantification with ascorbic acid enriched zooplankton. Aquacul Inter 11:81-93
- Keshavanath P, Renuka P (1998) Effect of dietary L-carnitine supplements on growth and body composition of fingerling rohu, (Labeo rohita) (Hamilton). Aquacu nutri 4:83-88
- Tewary A, Patra B C (2008) Use of vitamin C as an immunostimulant. Effect on growth, nutritional quality, and immune response of Labeo rohita (Ham.). Fish Physiol Biochem 34:251-259
- Fraser DR (2018) Evolutionary Biology: Mysteries of Vitamin D in Fish. In Vitamin D 1:13-27
- Ling-hong M, Xian-ping G, Jun X, Bo L, Ke-bao W, et al. (2015) Dietary vitamin D3 requirement of Wuchang bream (Megalobrama amblycephala). Aquacu 436:104-109
- Labh SN, Shakya SR (2016) Fruits of lapsi Choerospondias axillaris enhances ascorbic acid level in brain and liver of common carp (Cyprinus carpio L) during intensive aquaculture. IJCS 4:199-205
- Shiau SY, Hsu TS (1999) Quantification of vitamin C requirement for juvenile hybrid tilapia, Oreochromis niloticus× Oreochromis aureus, with L-ascorbyl-2-monophosphate-Na and L-ascorbyl-2-monophosphate-Mg. Aquacu 175:317-326
- Shalaby AM, Khattab YA, Abdel Rahman AM (2006) Effects of Garlic (Allium sativum) and chloramphenicol on growth performance, physiological parameters and survival of Nile tilapia (Oreochromis niloticus). J Venom Anim Toxins Incl Trop Dis 12:172-201
- Hall FG, Gray IE (1929) The haemoglobin concentration of the blood of marine fishes. J boil Chem 81:589-594
- Islam MS (2012) Impact of water quality on fish growth and production in semi-intensively managed aquaculture farm. Bangladesh J Environ Sci 23:108-113
- Gomez KA and Gomez A (1984) Statistical Procedure for Agricultural Research-Hand Book. John Wiley & Sons, New York.
- Fry FEJ (1971) The effect of environmental factors on the physiology of fish. Fish physiology 6:1-98
[Crossref]
- Rahman MS (1992) Water quality management in Aquaculture BRAC prokashana. Dhaka-1212, Bangladesh.
- Mitra G, Mukhopadhyay PK (2002) Growth, Nutrient Utilization, and tissue biochemical changes in rohu, (Labeo rohita), fed with natural and prepared diets. J Appl Aquac 12:65-80
- Ali S (1982) Studies on the diurnal variation in physico-chemical factors and zooplankton in a fresh water pond. Bangladesh J Fish 2:15-23
- Ai Q, Mai K, Zhang C, Xu W, Duan Q, et al. (2004) Effects of dietary vitamin C on growth and immune response of Japanese seabass, (Lateolabrax japonicus). Aquacu 242:489-500
- Sahu PK, Jena J, Pratap Das PC (2007) Nursery rearing of kalbasu, Labeo calbasu (Hamilton), at different stocking densities in outdoor concrete tanks. Aquacu Res 38:188-192
- Uddin MN, Rahman MS, Shahjahan M (2007) Effects of duckweed (Lemna minor) as supplementary feed on monoculture of GIFT strain of tilapia (Oreochromis niloticus). Progress agric 18:183-188
- Mazid MA (2009) Impacts of fish population density in the growth and production of carps in polyculture system. Department of Fisheries Management, Bangladesh 25:178-198
- Boyd CE (1990) Water quality in ponds for aquaculture. Albania Agricultural Experiment station, Auburn University, Alabama.
- Kohinoor AHM, Haque MZ, Hussain MG, Gupta MV (2000) Growth and survival of Labeo rohita, Labeo bata spawn in nursery ponds at different stocking densities. J Asiat soc Bangladesh Sci 20:65-72
- Nirod DB (1997) Effect on stocking density on the growth and production of mola (Amblypharyngodon mola) Department of Fisheries Management, Bangladesh Agricultural University, Mymensingh 42:1231-1235
- Apu JK, Rahman MS, Rashid H (2012) Effects of fish population densities on growth and production of fishes. Agricu 23:63-73
- Murhekar GH (2011) Assessment of physico-chemicalstatusof ground water samples in Akot city. Res j chem Sci 1:117-124
- Samad MA, Chowdhury P, Chatterjee SK, Rahman MT, Barman SC, et al. (2017) Growth response of threatened Labeo calbasu (Hamilton) fingerling based on stocking density in ponds. Int J Expt Agric 7:1-8
- Narejo NT, Salam MA, Sabur MA, Rahmatullah SM (2005) Effect of stocking density on growth and survival of indigenous catfish, Heteropneustes fossilis (Bloch) reared in cemented cistern fed on formulated feed. Pak J Zool 37:49-52
- Imtiaz A (2018) Effects of feeding levels on growth performance, feed utilization, body composition, energy and protein maintenance requirement of fingerling, rainbow trout, Oncorhynchus mykiss (Walbaum, 1792). Iran J Fish Sci 17:745-762
- Misra CK, Das BK, Mukherjee SC, Pradhan J (2007) Effects of dietary vitamin C on immunity, growth and survival of Indian major carp Labeo rohita, fingerlings. Aquac Nutr13:35-44
- Andrews JW, Murai T, Page JW (1980) Effects of dietary cholecalciferol and ergocalciferol on catfish. Aquacu 19:49-54
- Wang X, Kim KW, Bai SC, Huh MD, Cho BY, et al. (2003) Effects of the different levels of dietary vitamin C on growth and tissue ascorbicacid changes in parrot fish (Oplegnathus fasciatus). Aquacu 215:203-211
- Ibrahem MD, Fathi M, Mesalhy S, El-Aty AA (2010) Effect of dietary supplementation of inulin and vitamin C on the growth, haematology, innate immunity, and resistance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 29:241-246
- Gouillou-Coustans MF, Bergot P, Kaushik SJ (1998) Dietary ascorbic acid needs of common carp (Cyprinus carpio) larvae. Aquac 161:453-461
- Chen HY, Chang CF (1994) Quantification of vitamin C requirements for juvenile shrimp (Penaeus monodon) using polyphosphorylated L-ascorbic acid. J Nutr 124:2033-2038
- Ashraf M, Ayub M, Rauf A (2008) Effect of vitamin C on growth, survival and resistance to Lernaea infection in mrigal (Cirrhinus mrigala) fingerlings. Pak J Zool 40:165-170
- Soliman AK, Jauncey K, Roberts R J (1994) Water‐ soluble vitamin requirements of tilapia: ascorbic acid (vitamin C) requirement of Nile tilapia, Oreochromis niloticus (L.). Aquacu Rese 25:269-278
- Halver JE, Ashley LM, Smith RR (1969) Ascorbic acid requirements of coho salmon and rainbow trout. Trans Am Fish Soc 98:762-771
- Maheswaran R, Devapaul A, Muralidharan S, Velmurugan B, Ignacimuthu S, et al. (2008) Haematological studies of fresh water fish, Clarias batrachus (L.) exposed to mercuric chloride 2:49-53
- Nayak SK, Swain P, Mukherjee SC (2007) Effect of dietary supplementation of probiotic and vitamin C on the immune response of Indian major carp, Labeo rohita (Ham.). Fish Shellfish Immunol 23:892-896
- Hilton JW, Cho CY, Slinger S J (1978) Effect of graded levels of supplemental ascorbic acid in practical diets fed to rainbow trout (Salmo gairdneri). J Fish Res 35:431-436
- Tandel RS, Sharma A, Dash P, Bhat RAH, Deo A, et al. (2019) Dietary vitamin C supplementation on growth and haemato-biochemical parameters of Labeo rohita (Ham.) fingerlings. J Entomol Zool Stud 7:72-79
- Falahatkar B, Soltani M, Abtahi B, Kalbassi MR, Pourkazemi M, et al. (2006) Effects of dietary vitamin C supplementation on performance, tissue chemical composition and alkaline phosphatase activity in great sturgeon (Huso huso). J Appl Ichthyol 22:283-286
- Zhou Q, Wang L, Wang H, Xie F, Wang T, et al. (2012) Effect of dietary vitamin C on the growth performance and innate immunity ofjuvenile cobia (Rachycentro canadum). Fish Shellfish Immunol 32:969-975

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
