Simple Method for Estimation of Efficacy of Feed Enzymes
Cherkizovo Research Centre, 108805, Moscow, Russia
- Corresponding Author:
- Boris Sonichev
Cherkizovo Research Centre
108805, Moscow, Russia
E-mail: bsonichev@gmail.com
Received Date: November 01, 2021; Accepted Date: November 19, 2021; Published Date: November 26, 2021
Citation: Sonichev B, Shapovalov S (2021) Simple Method for Estimation of Efficacy of Feed Enzymes. J Anim Res Nutr Vol.6 No.11:120.
Copyright: © 2021 Sonichev B. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Today there is no single standardized method for determining the activity of NSP destroying enzymes. Each NSP enzyme manufacturer uses own approach as well as its analytical conditions (pH, temperature, substrate, etc.). In practice, the determination of enzyme activity shows activity of a particular enzyme, included in a commercial product. This creates a difficulty to navigate for consumers and to compare this variety of available products in the market. In this work Cherkizovo research centre suggested to use concept of “enzyme efficacy” and developed new method for its quantitative analysis. It was demonstrated that the end product of the enzymatic reaction can act as a working indicator of the enzyme efficacy. The authors demonstrate simple method for estimation of enzyme efficacy and suggest using it as preliminary result when assessing the possibility of using them for a particular diet and various feed raw materials.
https://linktr.ee/marmaris1 https://www.divephotoguide.com/user/marmaris1/ https://artmight.com/user/profile/2408931 https://allmyfaves.com/marmaris1 https://www.fimfiction.net/user/628252/marmaris https://www.drupalgovcon.org/user/568666 https://www.roleplaygateway.com/member/marmaris1/ https://www.kickstarter.com/profile/825997479/about https://tapas.io/sam659667 https://seedandspark.com/user/marmaris1 https://starity.hu/profil/386591-marmaris1/ https://www.informationweek.com/profile.asp https://nootheme.com/forums/users/marmaris1/ https://app.roll20.net/users/12308972/marmaris1-m https://www.360cities.net/profile/sam659667 https://fileforum.com/profile/marmaris1 https://wordpress.org/support/users/marmaris1/ https://www.hebergementweb.org/members/marmaris1.539143/ https://forum.cs-cart.com/u/marmaris1/ https://www.tntxtruck.com/User-Profile/UserId/12208 https://calis.delfi.lv/blogs/posts/205779-linkler/lietotajs/310821-marmaris1/ https://profile.ameba.jp/ameba/marmaris1/ https://www.avianwaves.com/User-Profile/userId/184770 https://engine.eatsleepride.com/rider/marmaris1 https://keymander.iogear.com/profile/53051/marmaris1 https://www.mifare.net/support/forum/users/marmaris1/ https://inkbunny.net/marmaris1?&success=Profile+settings+saved. https://www.diggerslist.com/64e0c4fad170b/about https://research.openhumans.org/member/me/ http://bluerevolutioncrowdfunding.crowdfundhq.com/users/marmaris1 https://www.cakeresume.com/me/marmaris1 https://educatorpages.com/site/marrmaris1/pages/about-me? https://gotartwork.com/Profile/marmaris1-marmaris1/253385/ https://www.facer.io/user/OGfo7VWrhR https://able2know.org/user/marmaris1/ https://www.techrum.vn/members/marmaris1.230749/#about http://foxsheets.statfoxsports.com/UserProfile/tabid/57/userId/145739/Default.aspx http://riosabeloco.com/User-Profile/userId/196075 http://phillipsservices.net/UserProfile/tabid/43/userId/245691/Default.aspx http://www.ramsa.ma/UserProfile/tabid/42/userId/962725/Default.aspx http://krachelart.com/UserProfile/tabid/43/userId/1242308/Default.aspx http://kedcorp.org/UserProfile/tabid/42/userId/72907/Default.aspx http://atlantabackflowtesting.com/UserProfile/tabid/43/userId/561626/Default.aspx https://www.intensedebate.com/people/marmaris12 https://rosalind.info/users/marmaris1/ https://wordpress.com/me https://photozou.jp/user/top/3342211
Keywords
Enzymatic hydrolysis; Enzymatic efficacy; Animal feed; Feed enzymes; Cherkizovo research center
Introduction
Nowadays, feed enzymes are commonly used in animals` nutrition. Enzymes aiming at the destruction of non-starchy polysaccharides (NSP-enzymes: xylanase, glucanase, and cellulase) are the second most popular in the world after phytase and the most popular in Russia. Other enzymes, such as protease, mannanase, pectinase, amylase, galactosidase are also in use.
Due to the widespread use of enzymes, the feed production market requires accessible, informative, and validated methods for evaluating enzymes in commercial products and premixes as well as in compound feeds. Such a routine study to determine the activity of enzymes should be an essential step to the control of the use and quality of enzyme production and application [1].
Enzyme activity is determined according to an approved method using a specific substrate, at a specific temperature and within a specified time interval. Enzyme activity is expressed in units of activity, which indicates the amount of enzyme required to release 1 mmol of monosaccharide in 1 min (μmol x min- 1). This indicator is applicable to determine the quality of the enzyme during production, the stability during storage and after granulation, and other aggressive effects on feed.
In the Russian Federation, several state standards have been adopted for determining the activity of xylanase, glucanase, cellulase, and amylase. GOSTs (Russia state standard specifications documents) are developed for various industries, in particular, for xylanase and cellulase for the pulp and paper and alcohol industries, as well as for the food industry. Also, GOSTs have been developed with the participation of All-Russian State Center for Quality and Standardization of Medicines for Animals and Feed (VGNKI) and Lekbiotech SPA to determine the activity of enzymes in animal husbandry in the production of compound feed. The following standards are recognized for food industry:
1. GOST 31488-2012. Enzyme products. Methods for determining the enzymatic activity of xylanase.
2. GOST 31662-2012. Enzyme products. Methods for determining the enzymatic activity of cellulase.3. GOST 34176-2017. Enzyme products. Methods for determining the enzymatic activity of endo-β-glucanase.
4. GOST 54330-2011. Enzyme products for the food industry. Methods for determining amylolytic activity have been developed for the food industry, but can also be used to determine the activity of amylase in compound feed.
Today there is no single standardized method for determining the activity of NSP destroying enzymes. Each NSP enzyme manufacturer uses own approach as well as its analytical conditions (pH, temperature, substrate, etc.). Therefore, each manufacturer gives own concept of the unit of activity of NSP enzymes [2].
The level of activity of enzyme products is most important for determining their viability. The type and dosage of the drug are selected based on the degree of the enzymatic activity (or the ratio of different activities). Different research laboratories, companies, and countries apply completely different methods for determining such activity. Currently, there is large number of both foreign and domestic commercial feed NSP-destroying enzyme products. They are obtained by biosynthetic processes of various microorganisms that synthesize extracellular enzyme complexes or individual enzymes. Complex multienzyme products determine the versatility of their action on various types of NSP and diets [3].
A wide variety of enzyme activity units that are used by different manufacturers is primarily due to conveniecy for each manufacturer to use “own" activity unit to standardize its enzymes during production. Obviously, the direct method for determining the activity ("activity in use") is the "in-vivo” test, that is, directly when feeding animals, however, this is not always possible, especially at the stage of development and manufacture of the enzyme product. Therefore, industrial enzymology in the production and commercialization of enzymes usually uses the value of activity determined "in-vitro”, in a biochemical laboratory without the use of living organisms [4].
This creates a difficulty to navigate for consumers and to compare this variety of available products in the market. Manufacturers indicate specific activities of enzymes, as a rule, guided by their concepts of units of activity and methods of their determination. The analytical methods proposed by the specifications include expensive substrates and standard samples, while the substrates in different methods may differ, and the standards have the claimed activities obtained by unknown methods. As a result, the proposed rather expensive activity analysis does not provide comparable results. Some authors offer relatively simple instrumental methods, such as determination of protein in a product. However, as multi-enzyme complexes of different activities are often used as feed additives, such methods provide only approximate data. In addition, there are objective differences between enzyme products of the same purpose, associated with the manufacturing method, in particular, with the nature and properties of the producers (fungi, bacteria), which is reflected in the conditions of the enzymatic reaction (pH, temperature, duration of hydrolysis). As a result, the consumer is only guided by the input norms recommended in the instructions for use, which are to be taken on faith [5-11].
In practice, the determination of enzyme activity shows activity of a particular enzyme, included in a commercial product. This activity indicates the "vitality" of the enzyme. Products with multi-enzyme composition are more complex to analyze. It is not clear if one should determine the activity of all enzymes included in the composition or the amount of the final product that accumulates as a result of the "enzyme-substrate" reaction.
The reaction product, i.e. monosaccharide, which is usually a reducer, can be determined in several ways. One of the generally accepted methods is the use of DNS reagent (3,5-dinitrosalicylic acid) [5,7].
Due to all of these reasons, we have a huge number of commercial products - multi-enzyme compositions and have no tool to adequately assess their activity or effectiveness. As a result, consumers (producers of poultry and eggs) have to conduct production trials to select the most efficient product on the market that would meet their price expectations and quality requirements. These production tests are associated with financial and labor costs, diverting the resources of the enterprise to side activities that are not related to production.
Some authors [12-14] in 2015-2016 conducted a comparative analysis of a large number of enzyme products using their single unit for xylanase, glucanase, and cellulase activity. However, the analysis also used some arbitrary unit of activity, which gives the consumer no evidence about the effectiveness of the enzyme.
Thus, the activity of enzymes in units can only be useful for assessing the viability of an enzyme or its presence, for example, in compound feed after granulation or extrusion, and there is no way to rely on units of enzyme activity in a production environment, where an enzyme can only be characterized by determining its effectiveness either by carrying out production tests, as is done now, or in “in-vitro” tests in conditions close to the gastrointestinal tract in terms of temperature and pH.
To sum up, the situation in feeding and fodder production is such that feeding specialists at compound feed factories, technologists and livestock specialists of livestock enterprises cannot rely on the methods proposed by them for assessing the activity of enzyme preparations, based on the determination the number of certain units of activity, as well as some, say, a single unit of activity that would have been established by someone. This situation today urges preliminary production tests of any enzyme products that had not been previously used in this enterprise, or relies on and apply the recommendations of colleagues, taking it at face value.
Experimental biochemistry, especially enzymology, still has needed to assess the units of enzyme activity since it is a fundamental concept in enzymology, which applies not only to feed but also to any other enzymes, primarily of endogenous nature. In a laboratory setting, from a scientific point of view, such techniques are acceptable for assessing enzyme activity.
These methods for determining the activity characterize the enzyme in terms of its activity, rather than efficacy. Activity, as mentioned above, is the work of the enzyme under "refined" conditions at a certain temperature, pH, in a specified time interval and with a chemically pure substrate, which shows the quality or, so to speak, its viability, considering the shelf life, thermal and storage stability.
Materials and Methods
Objectives of Experimental Method
The experimental method was developed by our group in Cherkizovo research centre. We have developed the concept of “enzyme efficacy”. Today standard methods are targeting at quantitative analysis of enzymes, required to produce 1 mmol of sugar. In our experiment we use quantitative analysis to estimate the amount of free sugar, produced by a certain enzyme.
The end product of the enzymatic reaction can act as a working indicator of the enzyme efficiency. The basis of the experimental method lies in the concept of “enzyme efficacy”, which is the ability of an enzyme to destroy the substrate with the release of monosaccharide(s) in the "field" conditions. In the first part of the experiment we have simulated field conditions as follows:
➢ Temperature close to that in the gastrointestinal tract of 39°С
➢ pH close to the pH of the gastrointestinal tract of 4.01 and 6.86
➢ Exposure time of 1 hour
➢ Complex substrate: crushed grain raw materials and their grain mixture as well as considering enzyme inhibitors.
In the second part of the experiment the pH was varying in the range of:
➢ 4.01 to 6.86
➢ 6.86 to 4.01
This was done in order to simulate the transition of the chyme to different parts of the gastrointestinal tract, where pH also changes medium from acidic to neutral and from neutral to acidic. This method yields more information on efficacy. For each tested enzyme we can predict:
➢ Its behaviour in specific conditions
➢ Its reaction on a specific raw material
➢ correlation between the results and structure of compound feed
For reference, the same raw material and the same conditions are used but without an enzyme product. Efficacy can be determined by feed tests through increasing the nutritional value of feed raw materials under the action of enzymes [14] in our modification.
Experimental design
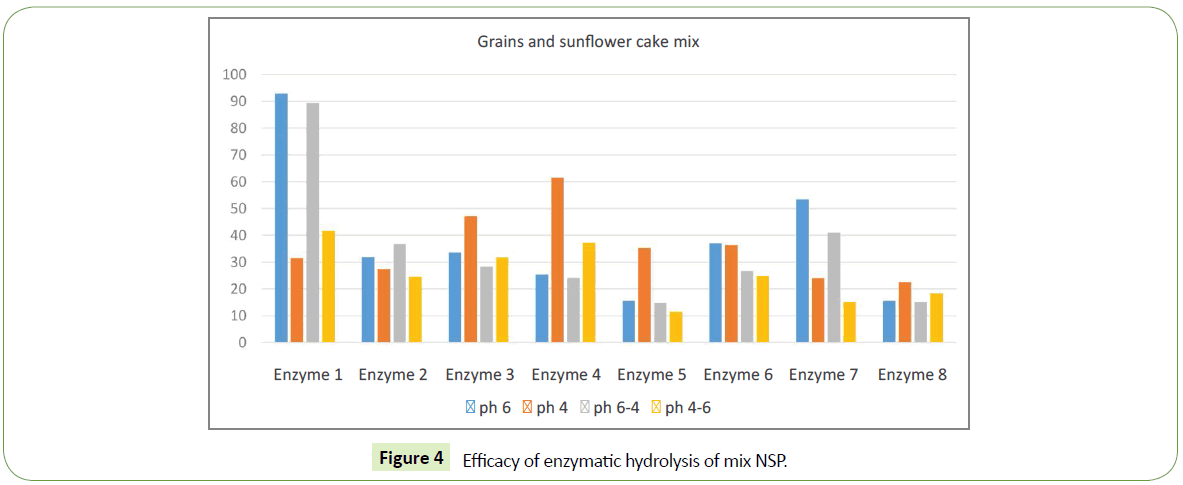
The experiment used grain milled with a sieve of 1 mm in diameter, embedded in a buffer with a pH 4.01 or pH 6.86 and incubated in a water bath at 39° for an hour; then the supernatant liquid after centrifugation was taken and the amount of sugars was investigated by the DNS reagent method at 540 nm. Then the pH changed from 4.01 to 6.86 and from 6.86 to 4.01 and again incubated for an hour at 39°C and again the amount of sugars in the supernatant was examined. As a control, we used the same milled grain, embedded in the same buffers but without enzymes. Efficacy was determined by the increase in isolated sugars under the influence of various multienzyme products relative to the control, expressed as a percentage. Control is the amount of released sugars in the control sample free of enzymes. The control was taken as 100%. Grains used in feeding were wheat, barley, corn and a mixture consisting of wheat - 35%, barley - 20%, corn - 30%, and sunflower cake - 15%. Summary of substrates and enzymes used in each experiment are given in Table 1.
| Substrate mix components | |
| Experiment 1 | 100% barley |
| Experiment 2 | 100% wheat |
| Experiment 3 | 100% corn |
| Experiment 4 | 35% wheat + 20% barley + 30% corn + 15% sunflower cake |
Table 1: Summary of the Experimental details and Medium used.
Results and Discussion
It was demonstrated that the end product of the enzymatic reaction can act as a working indicator of the enzyme efficacy. It is a simple, fast and reasonable way to estimate the enzyme efficacy that can be used both by customers and by food suppers. It yields clear and comparable values, which can give a preliminary result when assessing the possibility of using them for a particular diet and various feed raw materials.
Effect of substrates
All substrates used were in the same granularity, amount and humidity, thus the difference in the free sugar release should be only attributed to the changes in the experimental parameters: pH and enzymes used.
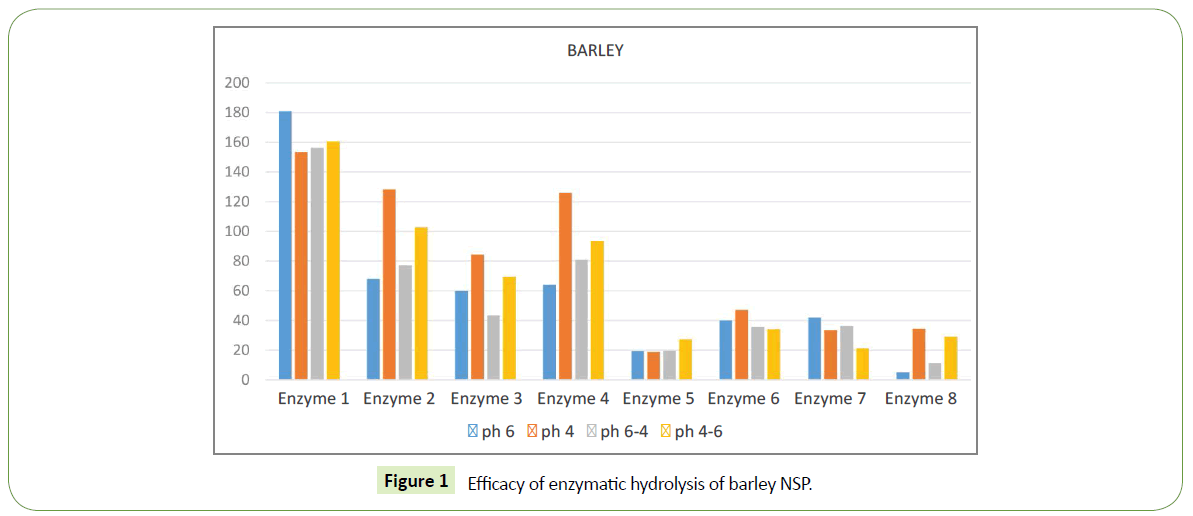
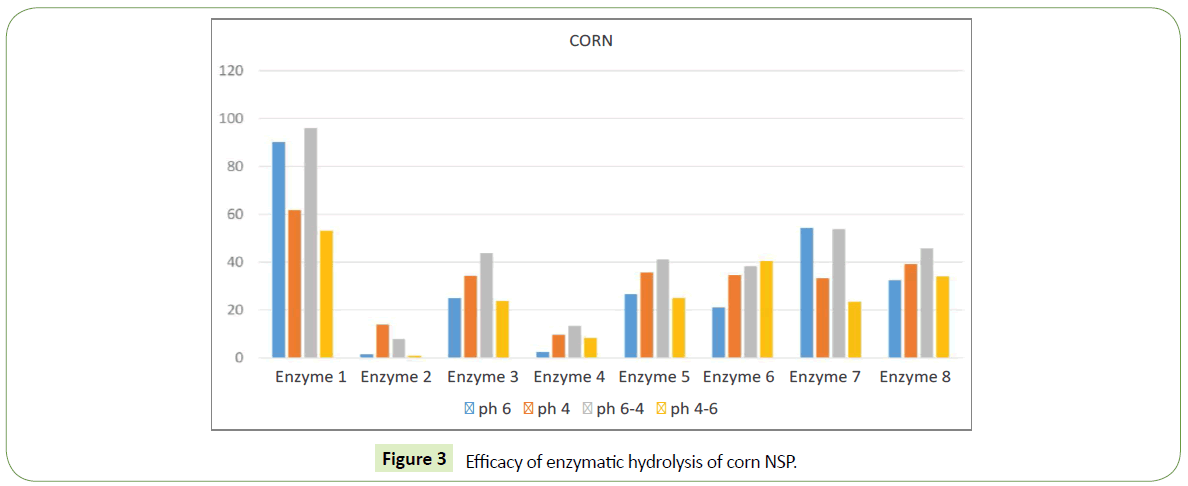
We identified significant difference in efficacy of enzymatic hydrolysis on different substrates. Figure 1 shows results for 100% barley substrate. The amount of isolated sugars released was highest (~180%) for this substrate compared to others (Figures 1-4). The lowest efficacy was demonstrated by corn substrate at the presence of Enzyme 2: almost zero. It is evident from the comparison the figures, that the efficacy of hydrolysis for all the four substrates (and we assume for all the commercially used combinations of those), is highly influenced by the applied enzyme.
Effect of Enzymes
A good benchmark for the method to work correctly is good correlation between effects of same enzymes on different substrates. The method is sensitive enough to draw even insignificant difference in enzymes efficacy. Enzyme 5 yields lowest amount of isolated sugars and Enzyme 1 – the highest. That is in good agreement with our expectations and previous studies.
Most of used enzymes have similar effect on amount of free sugar on each substrate used: enzyme 1 gives highest sugar outcome, followed by enzyme 2, enzyme 4, enzyme 6 and the lowest is enzyme 5. This order fits well in what we expected to see and what other authors reviled by other methods.
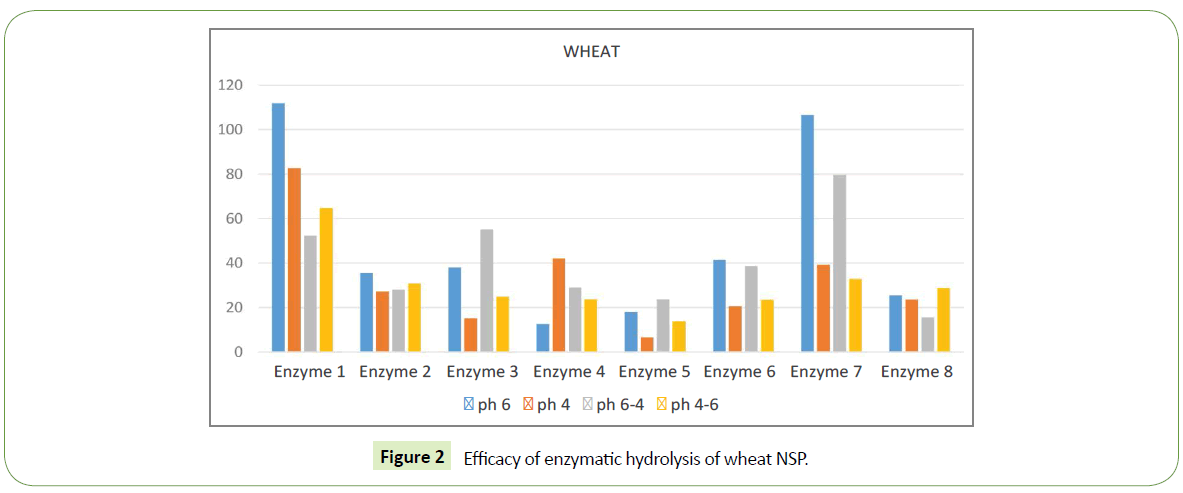
Enzyme 7 demonstrates different efficacy on few different substrates. For barley its quite low ~20-40%, which is 5 times less then isolated sugar released by Enzyme 1 in the same substrate, i.e. maximal value at this substrate. For barley this Enzyme 7 is one of the lowest by free sugar amount. When we look at wheat substrate enzyme 7 has much higher efficacy, almost the same as Enzyme 1. For comparison, Enzyme 1 yields ~55-110 %, while Enzyme 7 demonstrates 30-110 % of free sugar release.
In corn substrate the highest value of free sugar was observed at the presence of Enzyme 1 ~55-95%, while at presence of Enzyme 7 the value was 25 – 50%. This change in value should be attributed to different action of the enzyme in the presence of different substrates, rather than mistake in the experimental method.
The remaining enzymes were acting predictably in all substrates that demonstrate not only reliability of the evaluation method, but also its sensibility.
Effect of pH
Difference in pH used while experiment was aimed to simulate environment at different parts of the digestion system. On Figures 1-4 the pH values are the following:
➢ pH 6 and pH 4 – incubation at pH 6.86 and pH 4.01 respectively
➢ pH 6-4 – a change in pH from 6.86 to 4.01
➢ pH 4-6 – a change in pH from 4.01 to 6.86
Different pH of the environment has strong influence of the results. For example, when barley was used as substrate in the presence on Enzyme 1 the highest release of free sugar was noted at pH6 ~180%. The same experimental setting but at pH4 yields 155%, which is ~10 times lower.
Different substrates show different sensitivity to pH variations. For barley its reasonably low. The largest difference in free sugar release for barley was with Enzyme 4: pH6 ~65 %, pH4 ~130%, the difference of 2 times. For wheat the largest difference in free sugar release was with Enzyme 7: pH4-6 ~35%, pH6 ~110 %, which is a difference of almost 3 times.
It’s worth mentioning that no pH of environment guaranteed higher release of free sugar: at the presence of different enzymes the reaction of the substrate was different. Sometimes pH 4 yielded higher values, sometimes pH 6 did.
Conclusion
A method for changing the pH of buffer solutions is indicated as an imitation of the movement of chyme to different parts of the gastrointestinal tract. In this work, we used a well-known classical method using a DNS-reagent (reduction of 3,5-dinitrosalicylic acid to 3-amino-5-nitrosalicylic acid under the action of reducing sugars, which has a red-orange color, the color intensity is determined spectrophotometrically at 540 nm.
In this work, we used the "units" of the optical density of the color of the reagent in the supernatant liquid. The DNS-reagent was calculated according to the calibration graph built for glucose (Enzyme Nomenclature, of the nomenclature Committee of the IUB, N.Y., Academic Press, 1984; Miller G.L. (1959) Anal. Chem. 426-428).
The assay is do mimicking digestion as pH 4 is more common pH in the crop and proventriculum keeping in mind the buffering ability of the feed to change the pH upward (alkalinization), and pH 6,9 is more common pH at intestinal tract. More about overview of pH data at different segments of the gut of poultry shown in the table 6.1 on the page 136 of Enzymes in farm animal nutrition, by M. Bedford and G. Partridge. We took the average figures of pH.
The main goal is to obtain in invitro tests all reducing sugars obtained as a result of the destruction of various grain substrates of NSP. In our opinion, arabinoxylan and xylan may have the maximum water-holding capacity. The xylose itself, which is outside the polymer complexes, has a lower waterholding capacity, which causes a decrease in the viscosity of the chyme as an action of xylanases. The resulting composition of reducing sugars under the action of enzymes do determines their effectiveness. The higher the number of monosaccharides, the more available energy can be obtained and it decreases the viscosity of the chyme. The number of xylose also indicates the efficiency of the enzyme composition.
We found the results interesting because efficacy, i.e. the amount of isolated sugars varied depending on the multienzyme composition, the type of grain raw material or their mixture, as well as the change in the pH.
The results suggest that it is possible to select an effective multienzyme composition for a particular raw material, a particular diet structure before the production tests, and also confirm with a high degree of probability the matrix values of enzymes in energy since the fraction of additionally isolated sugars is nothing more than the countable additional available energy.
Laboratories today require simple and affordable ways to compare commercial drugs. Applied, routine enzymology today needs simple and cheap screening methods that can be available not only to large agricultural holdings, but also to small laboratories at feed mill plant or poultry farm."
References
- Aehle W. (2007) Enzymes in Industry: Production and Application. 3rd edition Wiley, New York 211-216.
- Bedford M, Partridge G. (2013) Enzymes in farm animal nutrition. 12-33.
- Bros J, Ward NE. (2007) The role of vitamins and feed enzymes in combating metabolic challenges and disorders. J Appl Poultry Res 16: 150.
- Enzyme Nomenclature of IUB (2009).
- Miller GL. (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31: 426-428.
- Moloskin SA. (2016) Feed enzymes from activity to efficiency. Analytical expertise and qualimetry 2: 73-74.
- Romero L. (2015) Influence of feed enzymes to the health of the intestine gut in poultry. Tsenovik 4: 78-83.
- Rosstandart. (2012) Enzymes drugs. Methods for determining the enzymatic activity of xylanase, Russian State standard 31488-2012.
- Rosstandart. (2012) Enzymes drugs. Methods for determining the enzymatic activity of cellulase, Russian State standard 31662-2012.
- Rosstandart. (2017) Enzymes drugs. Methods for determining the enzymatic activity of endo-β-glucanase. Russian State standard 34176-2017.
- Rosstandart. (2011) Enzymes drugs. Methods for the determination of amylolytic activity. Russian State standard 54330-2011.
- Shastak Y. (2016) Non-stach polysaccharides and methods for determination of their activity in animal nutrition. Analytical expertise and qualimetry 2: 69-72.
- Sinitsyn AP, Sinitsyna OA, Kondratyeva EG, Plokhov AY. (2014) The Activity of enzymatic preparations as a main criterion of their properties. Ptitsevodstvo 12: 36-40.
- Sinitsyn AP, Sinitsyna OA, Kondratyeva EG, Korotkova AS. (2016) Comparative assessment of different feed enzymes by a single unit of activity. Ptitsevodstvo 2: 35-38.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences