Controlling Hydrogen Sulfide Emissions during Poultry Productions
Ketwee Saksrithai, Annie J King
DOI10.21767/2572-5459.100040
Ketwee Saksrithai* and Annie J King
Department of Animal Science, University of California, Davis, 1 Shield Avenue, Davis, California, USA
- *Corresponding Author:
- Ketwee Saksrithai
Department of Animal Science
University of California, Davis
1 Shield Avenue, Davis, California, USA,
Tel: 530-752-3530
E-mail: ksaksrithai@ucdavis.edu
Received date: January 06, 2018; Accepted date: January 17, 2018; Published date: January 22, 2018
Citation: Saksrithai K (2018) Controlling Hydrogen Sulfide Emissions during Poultry Productions. J Anim Res Nutr 3: 1: 2. doi:10.21767/2572-5459.100040
Copyright: © 2018 Saksrithai, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Hydrogen sulfide (H2S) and other volatile sulfur compounds (VSC) have received a great deal of attention as gaseous emissions associated with poultry productions. These compounds, especially H2S, have low odor thresholds (10 ppb) and when managed improperly, higher concentrations of H2S negatively affect humans, poultry, and the environment. Primarily, odor emissions during poultry production depend on determinants such as sulfur containing compounds (cysteine and methionine) in feed and biological processes associated with their use/ production. Post feeding, as manure accumulates and during its storage, anaerobic decomposition of amino acids forms intermediate sulfur-containing compounds that ultimately form VSC. To manage poultry waste properly, it is important to have an understanding of determinants of H2S emissions, associated microorganisms, as well as their interactions. Promising areas of research to reduce odor emission include feed supplementation (additives, prebiotics, and probiotics); manure manipulation (pH, moisture, and it microbial population); housing types; ventilation rates; and bio filters. The most promising singular methods to reduce 100% H2S emissions are probiotic supplementation in feed, sawdust in manure, or a biofiltration system. Where cost and equipment availability may be prohibitive, combined methods (assuming additive effects) of fibrous byproducts and manure moisture control via microorganisms or oil addition can also reduce 100% emissions as well. More investigations should focus on these single or combined methods in commercial poultry production.
Keywords
Poultry; Volatile sulfur compounds (VSC); Hydrogen sulfide (H2S); Probiotic supplements; Bio filters
Abbreviations:
CO2: Carbon Dioxide; H2S: Hydrogen Sulfide; H2SO4: Sulfuric Acid; NH3: Ammonia; Ppb: Parts Per Billion; Ppm: Parts Per Million; S: Sulfur; SO2: Sulfur Dioxide; SO4: Sulfate; SRB: Sulfate- Reducing Bacteria; Vscs: Volatile Sulfur Compounds
Introduction
In 2017, the National Agricultural Statistics Service reported that 7.73 billion table eggs were produced by 311 million layers in the US [1]. An increase in egg production is needed for the higher demand in the US and emerging economies around the world. High egg production is accompanied by a high accumulation of manure leading to complaints from neighbors living in close proximity to layer operations. Hydrogen sulfide (H2S), one of the volatile sulfur compounds (VSC), has received a great deal of attention as one of the gaseous emissions associated with animal feeding operations because of its low odor threshold (H2S=10 ppb) and its negative impacts on human and animal health and the environment.
The critical negative effects of H2S on humans and the environment have been the subject of other reports [2-6]. Here, we focus known effects of H2S on poultry, on determinants of H2S during poultry production, and various methods for control or prevention.
Negative effects for poultry
Only three studies of direct H2S toxicity on poultry have been published. Klentz and Fedde [7] studied the respiratory response of White Leghorn chickens to acute concentration of H2S (0, 0.05% 0.2%, 0.3%, and 0.4%). At 0.05%, there were no significant differences in tidal volume and respiratory frequency compared to the control group. At 0.2% and 0.3%, birds had an increase in respiratory frequency but returned to normal within 30 minutes after H2S exposure. All birds died within 15 minutes at 0.4% H2S inhalation; this is equivalent to 4000 ppm. The researchers noted that chickens are less sensitive to H2S than mammals, 500-1000 ppm leading to death. In the same study, they also examined the response of intrapulmonary CO2 receptors to varying H2S concentration (0.035-0.1% H2S). H2S caused an increase in intrapulmonary CO2 receptors’ discharge frequency and an increase in vertical sternal movements. This increase in discharge frequency inhibited carbonic anhydrase in the central respiratory neurons which led to apnea [7].
Kocaman et al. [8] observed that the concentrations of CO2, NH3, H2S, relative humidity, and temperature in winter and spring are significantly different from summer and fall. Moreover, researchers found that an increase in CO2 (950.0-1623.1 ppm), NH3 (10.5-16.46 ppm), and H2S (1.75-7.0 ppm) in poultry houses can decrease the feed conversion ratio (from 1.79 to 2.18 kg feed consumed per kg egg produced). The effect seems to be caused by a combination of the different gases and the condition of the poultry house rather than the effect of a single component.
Another study assessed the effect of only H2S on the performance of broiler chickens. Each treatment was in an environmentally controlled chamber with 0 mg/kg H2S in weeks 0-6 as a control; 3 treatments ranging from 2, 4, and 8 mg/kg H2S during weeks 0 to 3 and 3, 6, and 12 mg/kg of H2S during weeks 3 to 6. Results showed that H2S had negative effects on broiler performance, resulting in an increase in production cost. From weeks 0 to 3, average daily intake and body weight increased and the feed: gain increased as H2S concentration increased. The highest concentration of H2S (12 mg/kg) resulted in a significant decrease in carcass yield and a significant increase in the rate of water loss in breast and thigh. This result correlated with a decrease in pH values of breast and thigh. The researchers suggested that there should be less than 2 mg/kg of H2S in the broiler houses from weeks 0 to 3 and less than 6 mg/kg of H2S from weeks 4 to 6 for healthy broiler production [9]. Overall, researchers examine negative effects of ammonia on poultry have found detrimental effect of H2S along or in combination with other gases when it reached 1.75-7.0 ppm.
Determinants of H2S emission
One approach to control H2S is to understand the role of primary determinants such as S-containing amino acids, associated biological processes, microorganisms, and resulting interactions.
Amino acids in feed: Methionine is one of 13 essential amino acids required for growth of poultry [10,11]. Due to low methionine in plant products, it is the only amino acid that must be synthetically produced in a form of DL-methionine or methionine hydroxyl analogue (MHA) to add to poultry diets [12,13]. Of 18.8% crude protein, 0.38% is methionine. Thus, the total S required by chicken is approximately 4.5% of the total protein [10]. Chavez et al. [14] investigated the effect of different methionine sources (liquid MHA and DL methionine, dry MHA and sodium methioninate aqueous solution) and concluded that various methionine sources gave rise to the different odor profiles, varying concentrations of H2S, COS, CH3SH, CH3SSCH3, CH3S3CH3.
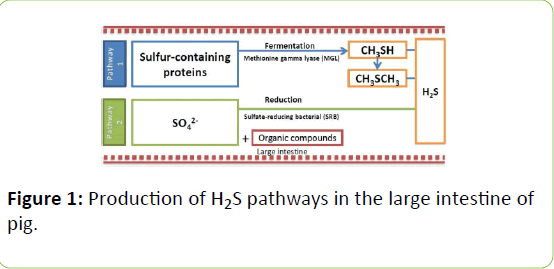
Biological process associate with sulfur amino acids: There are two possible pathways to form H2S and CH3SH in animalsbacterial degradation of the S-containing amino acids and the bacterial reduction of sulfate ions (SO42-) (Figure 1). The digestive pathway is for pig, a monogastic animal like chicken [15].
Microorganisms anaerobically decompose S-containing amino acids (cysteine/cystine and methionine) forming intermediate Scontaining compounds that ultimately form H2S and other VSC [16-19]. The enzyme responsible for this pathway is methionine gamma lyase, which is present in some organisms from archaea to bacteria to plants [20]. H2S, along with pyruvate and NH3, can also be released from the enzyme cystine desulfhydrase, catalyzing the α, β-elimination of L-cysteine [21]. Some of the Lactobacillus species, such as Lactobacillus plantarum, Lactobacillus casei, Lactobacillus acidophilus, and Lactobacillus brevis, were found to produce H2S by this pathway if grown on peptone iron, triple sugar iron and Sulfide-Indole-Motility agars [22].
More specific bacterial degradation of complex organic matter is through a branch of strictly anaerobic genera of Deltaproteobacteria, sulfate-reducing bacteria (SRB) such as Desulfovibrio [23], Desulfobacter [24], Desulfococcus, and Desulfonema [25]. This pathway has also been found in Campylobacter [26], Escherichia coli [27-30] and Salmonella [31-33]. These SRB use hydrogen and organic compounds for growth while reducing SO42- to H2S/HS- in the process. These redox reactions (1-3), where CH2O represents a generic organic carbon compound, depict the outcome.
SO42- + ATP → PPi + APS (1)
APS + 2CH2O +2e- → SO32- + 2H++ AMP+ 2HCO- (2)
SO32- + 6e- + 8H+ → H2S + 3H2O (3)
SRB may also use other volatile fatty acids such as acetate, propionate, butyrate, and lactate which serve as the final electron acceptor during cellular respiration providing energy and promoting the growth of these bacteria [25,34,35].
There is evidence of H2S production in the caecum of the chicken via microorganisms [36]. Gong et al. [37] identified bacteria present in the mucosa of chicken ceca using 16S rRNA. They found the chicken cecal environment to be highly diverse having butyrate-producing bacteria, which are closely related to Fusobacterium prausnitzii, as one of the largest groups among 116 cloned sequences. They also identified other bacteria such as Clostridia, Enterococcus cecorum, Escherichia coli, Lactobacilli, and Ruminococci. Basic et al. [38] reported their findings on proteins in Fusobacterium spp. that are involved in the production of H2S from cysteine. The most abundant enzyme detected was cysteine synthase which is involved in cysteine metabolism. Endogenous H2S production occurs as a reversible reaction of cysteine synthesis. Serine sulfhydrase, isolated from chicken liver, is known to catalyze the reversible reaction between H2S and serine to produce cysteine and water [39]. Ultimately, when investigating ways to negate the negative effects of H2S, it is important to account for naturally occurring endogenous sources.
Feed amendment with byproducts: Many investigators have assessed strategies to lessen production of H2S during storage of manure at its source (feed). These strategies include control of dietary S amino acids by addition of various byproducts and inclusion of phytobiotics, prebiotics, or probiotics to minimize the amount of leftover S in manure.
When investigating the effect of feed manipulation on reduction of H2S, literature of swine and broilers dominate that of layers. Results of swine research demonstrated the potential of reducing dietary S-containing amino acids and SO42- to reduce H2S emission [40]. Kendall et al. [41] provided reduced crude protein (from 11.5 % crude protein in the control to 8.25% in the treated groups) diets with 5% soybean hulls, high-available phytate corn, phytase, and reduced mineral SO42- for six weeks to determine the effect on pig growth performance, NH3, H2S, odor examined, and nutrient excretion. There was a reduction in concentration of both NH3 (48.7%) and H2S (48%) at week six.
Jiao et al. [42] supplied varying amount of dietary methyl sulfonyl methane (MSM, at 0%, 0.05%, 0.10%, and 0.20%) in order to examine the effect on broiler performance and gas emission. They found a linear trend for H2S reduction (P=0.09) with greater addition of MSM in the diet.
The effect of different dietary fat sources was evaluated for growth performance, excreta microbiology and noxious gas emission in broilers. The two fat sources were halal tallow and a combination of tallow and lard. The investigators found no significant difference in H2S reduction between the two fat sources during the 5-week study [43]. Researchers examined the same parameter for different treatments. The four treatments were (1) chicken fat, (2) tallow, (3) tallow and lard, and (4) pork fat/lard. Soybean was the control. NH3, H2S, and SO2 emissions were significantly lower in diets with soybean oil and chicken fat compared to others [44].
Sharma et al. [45] found that providing similar calculated digestible methionine plus cysteine (7.3 g/kg in wheat and canola seed diet vs. 7.0 g/kg in wheat-corn without canola seed control diet) resulted in a higher concentration of CH3SH from the diet with canola seed compared to that of the control. The researchers suggested that the significant difference was likely due to difference in moisture content. Higher moisture content produces more CH3SH caused by increased anaerobic degradation. Sharma et al. [46] also reported a significant positive correlation between litter moisture with CH3SH, H2S, CH3SCH3, trimethyl amine, phenol, indole, and skatole.
When Wu-Haan et al. [47] fed diets contained 6.9% of CaSO4 zeolite mixture to layers, they reported an increase in H2S concentration from laying hens manure at different ages. The average H2S daily emission over three weeks for the treated diet was significantly (P< 0.01) higher (4.08 mg/bird) than that of the control diet (1.32 mg/bird). Researchers suggested that the acidifying effect of CaSO4 contributed to the increase in H2S emission. Findings from another study using zeolite also showed zeolite in poultry manure lowered the concentration of other volatile compounds but increased VSC. The decrease in pH caused the noted change [48].
Wu-Haan et al. [49] investigated distillers dried grain plus soluble (DDGS), a by-product of corn from the beverage and alcohol industries, for its capacity to reduce H2S emissions. They investigated the effect of varying amounts of DDGS (0, 10, and 20%) in the diet on air emissions and laying hens performance. Each diet contained 0.22, 0.27, and 0.42% of S, respectively. Adding DDGS to the diet showed no significant effect on layer performance but a significant reduction in emissions. Daily emissions of NH3 and H2S from 21- to 26-week-old laying hens decreased at the 20% DDGS inclusion rate.
Chlorine dioxide has been investigated as a dietary supplement to reduce gaseous emission without affecting broiler performance. Addition of 0.05% and 0.1% chlorine dioxide resulted in an antimicrobial activity against Escherichia coli (in ileum and cecum) and Salmonella Typhimurim (in cecum). The reduction in these two sulfate-reducing bacteria may explain the reduction in H2S emission, significantly lowered at three hours of fermentation with 0.05% chlorine dioxide. The emission of CH3SH was significantly lowered starting at the 0 hour of fermentation for both 0.05% and 0.1% chlorine dioxide [50]. The highest H2S reduction rate (at 62.5%) of the feed additive was chemical addition of 0.05% chlorine dioxide.
Microorganism supplementation as feed amendment: Animals’ microflora need to be stable in order for improvement in feed efficiency and effective dietary nutrients utilization. Feed supplement, such as phytobiotics, prebiotics and probiotics should be considered for stabilization or improvement of the microflora community [51]. Phytobiotics, or phytogenics, are herbs, spices and plant extracts (essential oil) used in human traditional medicine [52]. In recent years, phytobiotics have been used as alternatives to antibiotic growth promoters for beneficial effects such as higher feed intake, anthelmintic (antiparasitic), antimicrobial, coccidiostatic, and immunostimulating properties [53].
Use of phytobiotics: A dietary phytogenic feed additive, extracted from Korean pine, has been reported to significantly reduce NH3 emission but no significant difference was found for total CH3SH, H2S and acetic acid. However, there was a significant positive correlation for reduction of all excreta gas emission and higher phytoncide supplementation [54].
A by-product of Punica granatum L. (pomegranate) has been used to investigate the effect of growth performance, noxious gas emission and economic efficacy in broilers. NH3 and H2S reduction were both significant, but not SO2. Optimal reduction of NH3 (37%) was found in the diet with 2.0% byproduct whereas optimal reduction of H2S (86%) was found in the diet with 0.5% by-product [55]. The same group of researchers investigated the effect of this byproduct on fecal growth performance, microbiology, and noxious gas emission in broilers. Broilers were fed varying amounts (0, 0.5, and 1%) of the byproduct for 35 days. Both levels of byproduct significantly reduced NH3 emission at 12, 24, and 48 hours. Significant H2S reduction was observed in 0 hour with 1% byproduct. Significant CH3SH reduction was observed at 0, 3, and 48 hours of incubation with increasing levels of the byproduct [56].
A combination of exudates of Lactobacillus plantarum - fermented Gynura procumbens, Saccharomyses cereviseae - fermented Rehmannia glutinosa, and Bacillus licheniformis - fermented Scutellaria baicalensis were investigated for their effect on broiler performance. Diets included varying amounts (0, 0.05, 0.1, and 0.2%) of the fermented product for 35 days. NH3 emission was significantly lower compared to the control diet. Investigators found no significant reduction in both H2S and total CH3SH emission but a significant linear correlation between the amount of H2S and total CH3SH produced and the amount of fermented product added [57].
Use of prebiotics: Prebiotics are non-digestible food ingredients that promote the growth of the host's beneficial microflora [58,59]. Zhao et al. [60] explored the effect of levan fructan supplementation on broiler performance microflora and excreta noxious gas emission. NH3 was significantly lowered by the addition of the supplement at 0.25% and 0.50% fructan (P<0.013). The higher amount of fructan did not improve the emission reduction efficiency. H2S and acetic acid gas emissions were not significantly different from that of the control diet.
Supplementation of lactulose, a non-digestible carbohydrate used in stimulating the growth of Lactobacillus, has been investigated to improve broiler performance contrary to finding of Zhao et al. [60]. NH3, H2S, and acetic acid gas emissions were decreased (P<0.05) in diets with 0.1% and 0.2% lactulose compared to the control diet. As well, only the 0.2% lactulose diet had a significant increase in excreta Lactobacillus and a significant decrease in Escherichia coli compared to the control diet [61]. While many researchers have investigated the use of prebiotics in broiler productions, others have also examined on the use of probiotic as discussed below.
Use of probiotics: Probiotics are live bacteria that, when ingested, may benefit the host by improving digestion [62]. Because in commercial poultry production chicks are separated from layers, the opportunity to transfer microorganism from the layer’s feces to young chicks to improve their digestion is reduced. Other possible microorganisms that can be offered to chicks to serve a similar function have been proposed. Mainly, Lactobacilli have been used as probiotics because they are predominantly found in the chicken’s crop epithelial cells [63]. Lactobacillus organisms, endogenous in chicken as well as humans, are ubiquitous in nature. Research results provided information on the intestinal benefit of single-strain probiotics (Bacillus cereus, Bacillus licheniformis, Bacillus subtilis, Enterococcus faecium, Pediococcus acidilactici, Lactobacillus farciminis, Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus plantarum, Streptococcus infantarius and Saccharomyces cerevisiae) for livestock and poultry [64]. Lactobacillus rhamnosus, alone, has been reported to reduce H2S production in vitro under both aerobic and anaerobic conditions [65].
There is little information about in vivo investigations on the effect of probiotics in poultry production; that which is available has focused on broilers. Jeong and Kim [66] determined the effect of spore supplementation of Bacillus subtilis on broiler performance and noxious gas (H2S and NH3) emission. This 5- week study used 1.0 × 109 cfu/g of Bacillus subtilis. The diets were 0, 300, and 600 mg of Bacillus subtilis/kg feed. There was no significant effect on the reduction of H2S. In another study, these investigators ascertained the effect of astaxanthin (a carotenoid pigment produced by a yeast species, Phaffia rhodozyma) on the same parameters and found the same nonsignificant reduction of H2S [67]. In a different study, Zhang and Kim [68] determined the effect of probiotic (Enterococcus faecium) supplementation with two levels of energy (2,700 or 2,800 kcal/kg, metabolizing energy) diet on Hy-Line brown layers. They also found no significant reduction in H2S emission or total CH3SH from freshly collected manure which was allowed to ferment for 30 hours in a sealed container before sample collection from the headspace.
In contrast, Lan et al. [69] investigated the effect of Enterococcus faecium on growth performance, excreta microbiota shedding, and noxious gas emission in broilers. They used varying amount of Enterococcus faecium in the treated diets (0, 0.05, 0.10, and 0.20%). At day 7, only H2S emission was significantly reduced compared to that of the control diet (P<0.001), but the amount of Enterococcus faecium did not have a significant effect. At day 35, emissions of NH3, H2S, and total mercaptans were significantly reduced compared to the control at 0.20% level (P= 0.002, 0.001, and 0.013, respectively). Lactobacillus was not significantly increased but Escherichia coli were significantly lowered at 0.10% and 0.20% Enterococcus faecium at day 7. At day 35, Lactobacillus was significantly increased at all levels with significantly lower level of Escherichia coli at 0.05% and 0.20%.
Zhang et al. [70] found a significant reduction in H2S concentration (37.9%) using only 105 cfu/kg of Bacillus subtilis alone compared to the control diet. Additionally, Sharma et al. [71] found a significantly lower H2S concentration (up to 29.9% reduction) in the litter from birds fed high crude protein with probiotic (Bacillus subtilis) added compared to other diets (high crude protein alone, high crude protein with antibiotic, and high crude protein with saponin at 26, 24, and 23 for starter, grower and finisher diet). However, the decrease in H2S concentration was not significantly different from that of the low crude protein diet (at 21, 19.5, and 18.4 for starter, grower, and finisher diet). The researchers noted the correlation between H2S and moisture content (r=0.482, P<0.01).
Ahmed et al. [72] determined the effect of Bacillus amyloliquefaciens on growth performance, cecal microflora, NH3, and H2S emission of broilers provided with varying amounts of probiotic (0, 1, 5, 10, and 20 g/kg) for 35 days. The results showed a negative linear and quadratic effects on fecal emissions of H2S (P<0.001) with optimum effect at 5g/kg of feed. Other results also suggested a positive effect on bird health.
Additionally, multi-strain probiotics (Lactobacillus reuteri, Enterococcus faecium, Bifidobacterium animalis, Pediococcus acidilactici, and Lactobacillus salivarius) isolated from intestinal tract of healthy adult chicken has been used as supplements to improve broiler growth responses, digestibility and cecal microflora composition [73]. Reportedly, the use of multi strain probiotics is better than single strain supplementation [74]. A combination of four bacterial strains of Lactobacilli, consisting of Lactobacillus casei, Lactobacillus brevis, Lactobacillus buchneri, and Lactobacillus plantarum, has been shown to significantly reduce the malodor from the broiler house. VSCs such as CH3SCH3 and CH3S3CH3 were decreased [75]. An in-vitro study showed that Lactobacillus plantarum and Lactobacillus rhamnosus have anti-microbial activity against Clostidium Perfringens, bacteria known to reduce sulfite to the sulfide ion [76-78].
The effect of probiotic containing Bacillus, Lactobacillus, Streptococcus, Clostridium, Saccharomyces, and Candida species at a rate of 3 g/kg feed (107-8 cfu/g) on broiler performance and odor was investigated. The method for detecting gaseous compounds were performed by holding inspection tubes (Gastec Co., Japan) one meter above the ground. Investigators found a reduction in NH3, H2S, and CH3SH in both male and female broilers compared to the control groups. The researchers concluded that these bacteria had a beneficial effect for overall broiler performance [79].
In contrast, [80] found no significant reduction of H2S in manure fermented for 1, 3, and 5 days with the use of spraydried spore-forming bacteria at 2 × 108 viable spores/kg of Lactobacillus acidophilus, Bacillus subtilis, and Clostridium butyricum in the diet.
A non-significant effect of multi-strain complex probiotic was observed in a different study by Balamuralikrishnan et al. [81]. The researchers used two different commercially available types - Probiotic A [Bacillus coagulans (1 × 109 cfu/g), Bacillus licheniformis (5 × 108 cfu/g), Bacillus subtilis (1 × 109 cfu/g), and Clostridium butyricum (1 × 108 cfu/g)] and Probiotic B [Bacillus coagulans – (1 × 109 cfu/g), Bacillus licheniformis (5 × 108 cfu/g), and Bacillus subtilis (1 × 109 cfu/g)].
Two strains of Bacillus subtilis were used in conjunction when challenging broilers with Salmonella typhimurium to understand the effect on performance, blood profiles, intestinal Salmonella concentration, and noxious gas emission. The two strains of bacteria were as effective as using the antibiotic (virginiamycin) in lowering the intestinal concentration of Salmonella. However, only the NH3 emission was significantly lowered. CH3SH, H2S, and acetic acid emissions were not significantly different from the control [82].
Hossain et al. [83] investigated the effects of Bacillus subtilis, Clostridium butyricum, and Lactobacillus acidophilus on excreta noxious gas emissions in broilers. In this study, the probiotics were added to the feed. Diets were (1) control (antibiotic free diet), (2) ANT1 (enramycin 5 ppm added), (3) ANT2 (avilamycin 5 ppm added), (4) TSP1 (0.1% probiotic added), and (5) TSP2 (0.2% probiotic added). Investigators found no significant effect on reduction of H2S concentration.
Manure, manure pH, and manure amendment: As noted above, research on feed manipulation can reduce H2S emission in manure. Other researchers have focused on direct manipulation of manure.
Lin et al. [84] quantified the source of S from three different laying hen houses (conventional cage, enriched colony, and aviary) to be about 91.7% from feed and 8.3% from water. Of the total, 67.8%, 25.9%, 6%, and 0.3% was deposited in manure, egg, air, and chicken as body weight, respectively. However, Wu- Haan et al. [85] reported 57.1% S after manure clean out at the end of a 3-week study. Less frequent clean-out time resulted in higher loss of S into the atmosphere. This conclusion is in agreement with results indicating that total reduced S concentration in the air was generally at its highest on storage days 30 to 35 [86].
Amino acid compounds have been found in animal manure [87-89]. Banwart and Bremner [90] investigated the origin of the VSCs including H2S, CH3SH, COS, CH3SCH3, CH3SSCH3, and CS2, and found that all, except H2S, are released from the decomposition of S-containing amino acids in soils treated with sludge, manure, dried or fresh plant materials. The release continues for up to 44 days. Investigators further noted that H2S was not detected due to its quick sorption by soil, making detection in the air impossible [91]. This may also be a possible reason for low concentration of H2S detected in other poultry houses.
More variety of VSCs was found in fresh manure than in old manure [86]. However, based on comments from a panel of 10 volunteers, the concentration of H2S, or the rotten-egg odor, was more prominent in the dried manure than in fresh manure [92].
Gay et al. [93] compared the total reduced S, NH3, and other odor levels from various animal housing facilities and manure storage sites. Composting of laying hen manure ranked third in highest emission of total reduced S compare to other types of animal manure storage units. The measurement ranged from 1.35 to 370.0 μg/s/m2, having the highest variability (standard deviation=104 μg/s/m2 for n=19). The researchers noted that the high variability may have been due to the differences in sampling sites, including factors such as diets, manure management, the design of the storage unit, seasons, and the ambient air temperature.
Manure pH and manure amendment can aid in removal of unwanted odors from poultry houses. Manure pH plays a crucial role in emission of H2S. The following equation derived by Xue et al. [94], shows the relationship between H2S concentration and the H+.
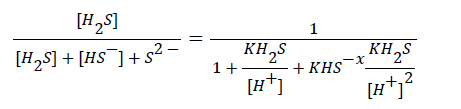
Where [H2S] is free H2S concentration (mol/L), [HS-] is HSconcentration (mol/L), K (H2S) is equilibrium constant for H2S (mol/L), [S2-] is concentration of S2- (mol/L), and K (HS-) is the equilibrium constant for HS- (mol/L) [95].
The equation shows that higher pH may reduce H2S emission into the atmosphere [96]. Sharma et al. [97] modeled the effect of pH on the H2S production by anaerobic sewer biofilm where multiple SRB species have been identified from the sewer biofilm [98]. The result of Sharma et al. [97] indicated that the maximum H2S production was at physiological pH (6.5-7.5). The S2- production was reduced outside of this range with up to 50% inhibition at pH 4.0 and pH 9.0. Free NH3 inhibited the effect on H2S production at high pH. The researchers were not able to determine the effect of low pH inhibition; however, they reported that acetic acid and other volatile fatty acids were not the cause.
A pilot-scale composting reactor showed the H2S reduction potential when adding sawdust to manure to improve the biodrying process; however, certain conditions must be met. The temperature must be more than 30°C above the ambient temperature. The moisture content should be between 30-40%. Exploring two conditions, the exhaust H2S of manure with sawdust was shown to be below the detectable limit (0.1 ppm, measured by a Gastech portable detector) compared to the manure without added sawdust (3-5 ppm) [99].
Gutarowska et al. [100] proposed to use a mixture of six strains of bacteria and one yeast (Bacillus subtilis subspecies spizizenii, Bacillus megaterium, Pseudomonas sp., Psychrobacter faecalis, Leuconostoc mesenteroides, Streptomyces violaceoruber, and Candida inconspicua) in the water as poultry manure deodorization. They found that the highest removal of volatile compounds (NH3, H2S, dimethylamine, trimethylamine, isobutyric acid) was caused by Bacillus subtilis subspecies Spizizenii, Leuconostoc mesenteroides, Candida inconspicua, and Psychrobacter faecalis. This surface application of bacteria removed NH3 and H2S from the exhaust gas by 20.8% and 17.5%, respectively. Moreover, there was a 45% reduction of protein and amino acids, particularly cysteine and methionine, after 24 hour of deodorization. A reduction of cysteine may explain the reduction in H2S concentration [101].
Matusiak et al. [102] further investigated the deodorizing capacity for the same six strains of microorganisms, enriched with two species of Lactobacillus plantarum. Mixtures of microorganisms in water were sprinkled on poultry manure with and without Yucca schidigera. Poultry manure was aerobically incubated in a sealed chamber with a flow rate of 2 L/min. The highest reduction in H2S concentration was the poultry manure with Yucca schidigera alone (64%), followed by poultry manure with microorganisms alone. This study also reported the benefit of the yucca extract to lower the concentrations of odorous compounds such as NH3, dimethylamine, H2S, isobutyric acid, and trimethylamine emitted from poultry manure. Yucca produces saponin that has been reported to inhibit microbial fermentation of protein [103].
Borowski et al. [104] was able to reduce NH3 and H2S from the exhaust air by 94% and 60%, respectively, after 2 days of deodorization using a combination of bacterial species (Pseudomonas fluorescens, Enterococcus faecium, Bacillus subtilis, Bacillus megaterium, Leuconostoc mesenteroides and Lactobacillus plantarum) on manure. The most effective method was 20% spray-dried microorganisms onto perlite and bentonite (2:8 ratios by weight) which was stored at room temperature (22 °C) for at least 5 months
A combination of spraying a water-oil mixture on manure and increased ventilation reduced H2S emission by 32% (from 13.2 to 9.0 ppm). The mixture was added at a rate of 80% sunflower oil and 20% water. The spraying area was 0.5 liter mixture for 100 m2 floor area. In addition to a reduction in H2S emission, this method also decreased temperature, relative humidity, concentration of dust, CO2 and NH3 [105]. The oil component most likely affects other parameters which lead to lowering H2S emitted in the house.
Quebracho tannins also have the ability to reduce H2S and methane gas emission by reducing the number of SRB and their metabolic activities. Stored swine manure was used in the experiment likely somewhat representative of poultry manure. It can be sprayed over manure or mixed with the liquid used to remove manure [106].
Packaged bacterial that can be added to manure to reduce H2S may become the norm in the future. Possibly, the combination of tannin-containing horticultural byproducts and packaged bacteria could be used to reduce SRB in manure.
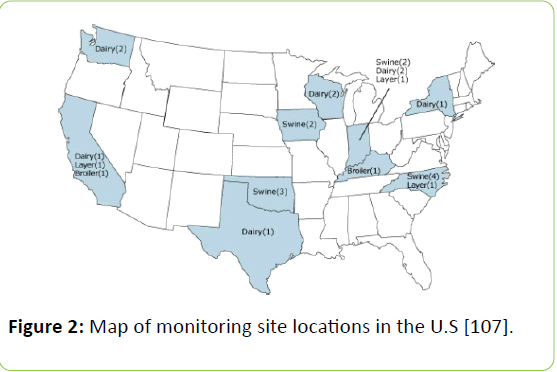
Housing: Housing types are critical as they often determine how manure is removed or stored over long periods of time. The National Air Emissions Monitoring Study [107] was funded by the Animal Feeding Operation (AFO) industry with the Environmental Protection Agency (EPA) to provide information about emissions of particulate matter, NH3, H2S, and volatile organic compounds from industries for swine, broilers, laying hens, and dairy. Out of 25 sites, only five were from poultry farms (3 layers and 2 broilers). The H2S emission data was collected from layer houses in North Carolina, Indiana, Kentucky, and California (Figure 2) [107].
Figure 2: Map of monitoring site locations in the U.S [107].
A detectable amount of H2S (19.7 ppb) was found in cagedhen high-rise layer houses (Figure 3) [108-112]. Layers were fed different types of feed to determine their efficacy in reducing NH3 and H2S. The control, a standard industry diet, produced 0.045 ppm of H2S as an average concentration over a two-year period. This was well above the detectable limit, as low as 0.01 ppm. Researchers noted that most of the odor in poultry houses comes from NH3 and H2S [109]. Almuhanna et al. [110] detected a lower average H2S concentration of 6.05 μg/m3 and 8.6 μg/m3 (=0.01 ppm) for two broiler houses. The maximum H2S concentration was 162.80 and 37.50 μg/m3.
Figure 3: Poultry housing: High rise with reverse stair-step [112].
However, the results are not clear because the two housing conditions were not specified.
A more recent study monitored two different type of poultry housing and found that manure-belt housing (Figure 4) [113]was 92% higher in emissions per animal unit (AU) and 78% higher in emissions per hen compared to high-rise houses (Figure 3) [112] (Table 1) [111].
Figure 4: Poultry housing: battery cage with manure-belt [113].
| State | Valid day (d) | Emission (gd−1AU−1) |
Emission (mg d−1hen−1) |
Reference | Type of house |
|---|---|---|---|---|---|
| Indiana | 84 | 0.484 | 1.52 | [107] | high-rise |
| Indiana | 314 | 0.5 | 1.55 | [115] | high-rise |
| Indiana | 313 | 0.4 | 1.26 | [115] | high-rise |
| California | 614 | 0.396 | 1.33 | [116] | high-rise |
| California | 632 | 0.374 | 1.2 | [116] | high-rise |
| North Carolina | 656 | 0.206 | 0.623 | [117] | high-rise |
| North Carolina | 652 | 0.237 | 0.694 | [117] | high-rise |
| Indiana | 276 | 0.506 | 1.46 | [118] | high-rise |
| Indiana | 296 | 0.442 | 1.28 | [118] | high-rise |
| Weighted mean | 0.355 | 1.101 | - | - | |
| Indiana | 634 | 0.679 | 1.95 | [111] | Manure-belt |
| Indiana | 624 | 0.685 | 1.96 | [111] | Manure-belt |
| Weighted mean | 0.682 | 1.955 | - | - | |
| 1H2S concentrations were recorded by pulsed fluorescence H2S analyzers. Ventilation rates were calculated from the fan monitoring system. Adapted from Ni et al., [111]. | |||||
Table 1: Daily means of H2S emissions 1 from nine reported laying hen houses using the same measurement method in the U.S.
| Determinant | Criteria | Conclusion | Quantifiable change | Reference |
|---|---|---|---|---|
| Pre-excretion strategies | ||||
| Dietary Fat Source | halal tallow vs. haram lard vs. chicken fat | decrease with chicken fat | 49% | [44] |
| By-product | DDGS1 (0, 10 and 20%) | decrease with 20% DDGS | 58% | [49] |
| Feed Additive | Chlorine dioxide (0.05, 0.1%) | decrease with 0.05% CIO2 | 62.50% | [50] |
| Phytobiotics | Punica granatum L. (0, 0.5, 1.0, 2.0%) | decrease with 0.5% | 86% | [55] |
| Punica granatum L. (0, 0.5, 1.0%) | decrease with 1.0% | 33% | [56] | |
| Prebiotics | Lactulose (0, 0.1, 0.2%) | decrease with 0.1, and 0.2% lactulose | 50%, 52.9%, respectively | [61] |
| Probiotics | ||||
| Single strain | Bacillus amyloliquefaciens (0, 5, 10, 20 g/kg feed) | decrease | 87.70% | [72] |
| Multistrain | Bacillus, Lactobacillus, Streptococcus, Clostridium, Saccharomyces, and Candida species | decrease | 100% (up to 1 ppm lowered) | [79] |
| Post-excretion strategies | ||||
| Moisture | 20% sawdust | decrease with increasing aerobic condition | 100 % (5 ppm lowered) | [99] |
| Manure Amendment | Bacillus subtilis subsp. spizizenii, Bacillus megaterium, Pseudomonas sp., Psychrobacter faecalis, Leuconostoc mesenteroides, Streptomyces violaceoruber,enriched with two species of L. plantarum and Yucca schidigera | decrease | 64% | [102] |
| Pseudomonas fluorescens, Enterococcus faecium, Bacillus subtilis, Bacillus megaterium, Leuconostoc mesenteroides, and Lactobacillus plantarum | decrease | 60% | [104] | |
| 80% sunflower oil and 20% water mixture | decrease | 32% | [105] | |
| Bio filter | Pseudomonas putida | decrease | 96% | [126] |
| Thiocbacillus thioparus | decrease | 97.5-98.0% | [127] | |
| Thiobacillus thioparus with Nitrosomonas europaea | decrease | 95% | [128] | |
| Acidithiobacillus thiooxidans and Hyphomicrobium VS | decrease | 99.80% | [129] | |
| compost/wood chips | decrease | 47%-94% | [130] | |
| 1DDGS- Distillers dried grain plus soluble | ||||
Table 2: Summary table of H2S mitigation strategies.
A report on poultry housing in South Korea stated that caged layer houses tend to have the highest levels of NH3 and H2S compared to layer houses with manure belts and broiler houses.
The author suggested that the difference in ventilation system could be the cause of this trend. Caged layer houses use a mechanical ventilation system which is usually set below the recommended ventilation rate to lower cost, whereas, the manure belt and broiler house have natural ventilation [119].
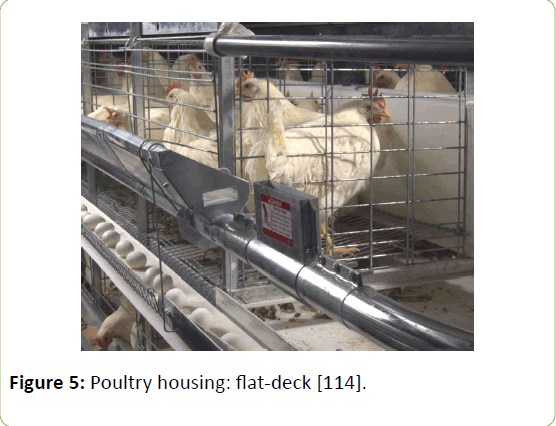
Leonard et al. [120] investigated the air quality in a broiler house for 20 minutes each week of the production cycle and found no detectable H2S using 10 ppb as the limit of detection. Broilers were raised in a wood-frame construction with earthen floors using short straw as litter. H2S production, along with gases such as CO2 and NH3, were measured from three different commercial laying hen barns by the same group of researchers. Barn A was a single-story house with individually housed hens stacked in three levels. Manure belts were used to collect droppings which were conveyed and elevated once a week to a manure spreader outside of the barn. Barn B was a double-story that had a deep-pit utilizing the lower half as the manure storage. Hens were lined up in three rows of doubly flat-deck cages (Figure 5) [114]. Manure was removed annually by a tractor with a front-end loader. Barn C, like Barn A, was a singlestory unit with three levels of stair-step cages. The droppings were scraped from the shallow manure pit monthly to a crossconveyor and elevated into a manure spreader. The researchers did not detect any H2S in barn A or C and only 30 ppb from barn B. They noted that this concentration was very low however, workers should be cautious when working in the barns especially during manure clean-out [121].
Figure 5: Poultry housing: flat-deck [114].
Guarrasi et al. [122] compared the occupational exposure of H2S in poultry, beef/dairy, and swine operations. They reported that poultry operations have the highest weighted mean H2S concentration (0.33 ppm) among the three different animal types. Further, they compared two different type of housing. The floor-based housing had the highest weighted mean H2S concentration (4.52 ppm) compared to the caged-based housing (0.04 ppm). The caged-based housing was a representative house for all layers whereas the floor-based housing represented the broiler operation.
Conclusions obtained from results of investigation indicate that the concentration of H2S found in poultry houses is relatively low. The danger is more pronounced when there is improper handling of wastes.
Ventilation rate and bio filters: As noted, proper handling of waste is critical when lessening the effects of H2S on workers. If manure is stored for any length of times, the ventilation rate should be clearly monitored. One of the ways to reduce VSC emission from poultry houses is to reduce the moisture content of the manure. A proper ventilation system can be used to control moisture and create appropriate indoor air quality [123]. Zhang et al. [124] investigated the combined effect of ventilation rate and the use of a super-plasma ionizing air purifier on the indoor air quality in broiler production. The different ventilation settings were 10 and 5 times per hour. The results showed that the 5 times per hour ventilation rate produced significantly higher concentration of H2S than the 10 times per hour ventilation rate.
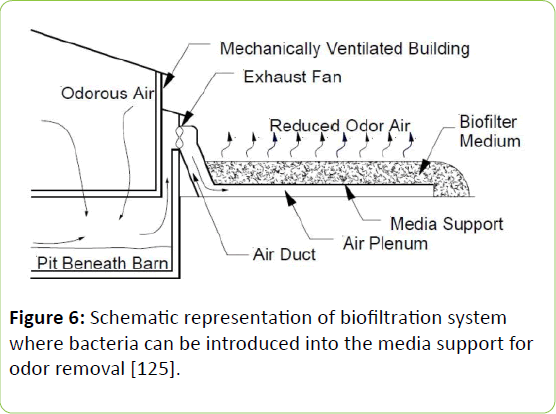
In addition to ventilation by fan, bio filters (Figure 6) [125] can be used to treat exhaust air in mechanically ventilated buildings by blowing through a media covered with a biofilm (containing bacteria). A species of bacteria with the ability to remove H2S from the exhaust air is Pseudomonas putida. Without causing acidification of the bio filter, this species of bacteria can convert H2S to mainly elemental S, allowing the microorganism activity to continue without much monitoring. When immobilized with calcium alginate, it was reported to remove up to 96% of H2S at 10 - 150 ppm with a flow rate of below 72 L/h [126].
Figure 6: Schematic representation of biofiltration system where bacteria can be introduced into the media support for odor removal [125].
Laboratory-scaled research was designed to show the capacity of immobilized Thiocbacillus thioparus as a biofilter to remove H2S under low-concentration conditions. High removal of H2S can be achieved with temperatures between 20-37°C (97.5-98.0%) and the flow rates of the inlet H2S concentrations are either 36 or 72 liters per hour. This species of bacteria oxidizes H2S to SO42-, elemental S, SO32-, and S2- [127]. Coimmobilization of T. thioparus and Nitrosomonas europaea was also found to be effective in reducing both H2S and NH3 emission. However, researchers found that H2S lowered the removal efficiency of NH3 but NH3 had no effect on removal of H2S [128]. Sercu et al. [129] used Acidithiobacillus thiooxidans and Hyphomicrobium VS in a two-stage biofiltration. Together, more than 99.8% H2S removal efficiency was achieved.
Sun et al. [130] further investigated the effect of bio filters in the removal of H2S with varying moisture content and reaction time, which is defined as the duration of contact time between air and bio filter media. When using compost/wood chips, the average removal rate of H2S varied from 47% to 94% with moisture content of 50% and gas retention time of 20 seconds being the highest removal rate. As noted by Bohn [131], bio filtration remains a promising field of research due to its minimal maintenance/cost and high efficiency; however, to our knowledge, there is no published research to date on the prevalence of bio filter use in the poultry industry.
Summary of methods used to effectively reduce H2S emissions
VSC and most especially H2S produced during poultry production is harmful to humans, poultry, and the environment. Understanding the chemistry and biological processes for production of H2S, employing mitigation processes from input (feed) to bio filters is necessary to greatly reduce emissions. Table 2 is an extensive summary of the best possible reported ways (above 30% reduction) to reduce VSC and H2S emissions discussed in this review. Thus, not all reduction methods are presented in the table.
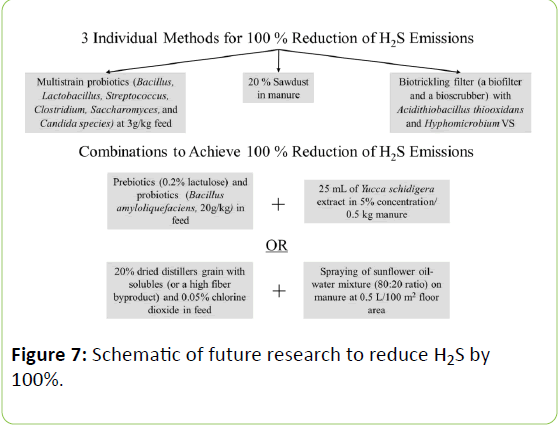
Table 2 was examined to determine recommendations for single or combined methods to achieve 100% reduction of H2S. As shown in Figure 7, this can be achieved by feeding multistrain probiotics, treating manure with sawdust, or installing a "biotrickling filter," combining a biofilter and a bioscrubber, in the poultry house. A combination of Bacillus, Lactobacillus, Streptococcus, Clostridium, Saccharomyces, and Candida species used as a probiotic in feed will not only decrease the amount of H2S released, but also promote and maintain a healthy gut microflora. Sawdust is used to lower the moisture content and to prevent the anaerobic decomposition of the undigested Scontaining amino acids. The biofiltration system using Acidithiobacillus thiooxidans and Hyphomicrobium VS, though proven only in the laboratory settings, relies on the ability of the selected strains of microorganisms to trap H2S as H2SO4 in the exhaust fan before the air is released to the surrounding area.
In locales where resources may be limited, other combined methods to reduce H2S emissions can be employed. The symbiotic effect of prebiotic(s) and probiotic(s) can promote the health of birds. If there is an additive effect, inclusion of 0.2% lactulose and 20g Bacillus amyloliquefaciens per kg of feed will reduce H2S, producing the desired effect of zero emissions. Or 5% concentration of Yucca schidigera extract adding at a rate of 25 mL per 0.5 kg manure can be used to eliminate H2S emissions from manure.
If microorganisms are inaccessible, byproducts such as DDGS (20%) or perhaps other high fiber ones can be added to feed in place of traditional corn-soybean meal. By supplying the antimicrobial activity of chlorine dioxide (0.05%) to the high fiber byproduct, an additional reduction of H2S can be achieved. Manure can also be sprayed with sunflower oil and water to greatly reduce H2S as well.
Conclusion
It is not likely that all possible ways to reduce H2S will be used at the recommended levels. While research to control H2S continues with broilers, more work should focus on their effects for laying hens especially during peak egg production at 30-40 weeks. With the recent advancement in microbial technologies, bio filtration and probiotics are promising areas of future research. Results of research, conducted in small laying hen houses under controlled conditions, can be scaled to industrial use, thereby affording more protection for animals, workers, and the environment, thus leading to a positive public acceptability of these very important agricultural operations.
References
- USDA (2017) U. S Department of Agriculture. Chickens and Eggs. National Agricultural Statistics Service.
- Legator MS, Singleton CR, Morris D, Philips DL (2001) Health effects from chronic low-level exposure to hydrogen sulfide. Arch Environ Health Int J 56: 123-131.
- Snyder J, Safir EF, Summerville GP (1995) Occupational fatality and persistent neurological sequelae after mass exposure to hydrogen sulfide. Am J Emerg Med 13: 199-203.
- Reiffenstein RJ, Hulbert WC, Roth SH (1992) Toxicology of hydrogen sulfide. Annu Rev Pharmacol 32: 109-134.
- Hendrickson RG, Chang A, Hamilton RJ (2004) Co-worker fatalities from hydrogen sulfide. Am J Ind Med 45: 346-350.
- USDL (2016) U. S Department of Labor. Bureau of Labor Statistics, Census of Fetal Occupational Injuries (CFOI).
- Klentz RD, Fedde MR (1978) Hydrogen sulfide: Effects on avian respiratory control and intrapulmonary CO2 receptors. Respir Physiol 32: 355-367.
- Kocaman B, Esenbuga N, Yildiz A, Laçin E, Macit M (2006) Effect of environmental conditions in poultry houses on the performance of laying hens. Int J Poult Sci 5: 26-30.
- Wang Y, Huang M, Mend Q, Wang Y (2011) Effects of atmospheric hydrogen sulfide concentration on growth and meat quality in broiler chickens. Poult Sci 90: 2409-2414.
- Almquist HJ (1952) Amino Acid requirements of chickens and turkeys. A review. Poult Sci 31: 966-981.
- NRC (1994) Nutrient requirements of poultry. Ninth Revised Edition, 1994, National Academy Press, Washington D.C.
- Baker DH (1986) Utilization of isomers and analogs of amino acids and other sulfur-containing compounds. Progr Food Nutr Sci 10: 133-178.
- Gordon RS, Sizer IW (1965) Conversion of methionine hydroxy analogue to methionine in the chick. Poult Sci 44: 673-678.
- Chavez C, Coufal CD, Carey JB, Lacey RE, Beier RC, Zahn JA (2004) The impact of supplemental dietary methionine sources on volatile compound concentrations in broiler excreta. Poult Sci 83: 901-910.
- Deng YF, Liao XD, Wang Y, Liang JB, Tufarelli V (2015) Prebiotics mitigate in vitro sulfur-containing odor generation in cecal content of pigs. Ital J Anim Sci 14.
- Kadota H, Ishida Y (1972) Production of volatile sulfur compounds by microorganisms. Annu Rev Microbiol 26: 127-138.
- Smet E, Van Langenhove H (1998) Abatement of volatile organic sulfur compounds in odorous emissions from the bio-industry. Biodegradation 9: 273-284.
- Mackie RI, Stroot PG, Varel VH (1998) Biochemical identification and biological origin in key odor components in livestock waste. J Anim Sci 76: 1331-1342.
- Kamoun P (2004) Endogenous production of hydrogen sulfide in mammals. Amino acids 26: 243-254.
- Sato D, Nozaki T (2009) Methionine gamma-lyase: the unique reaction mechanism, physiological roles, and therapeutic applications against infectious diseases and cancers. Life 61: 1019-1028.
- Ohkishi H, Nishikawa D, Kumagai H, Yamada H (1981) Distribution of cysteine desulfhydrase in microorganisms. Agr Biol Chem 45: 253-257.
- Lee BH, Simard RE (1984) Evaluation of methods for detecting the production of hydrogen sulfide, volatile sulfides, and greening by Lactobacilli. J Food Sci 49: 981-983.
- Postgate JR (1951) The reduction of sulphur compounds by Desulphovibrio desulphuricans J Gen Microbiol 5: 725-738.
- Widdel F, Pfennig N (1981) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol 129: 395-400.
- Muyzer G, Stams AJM (2008) The ecology and biotechnology of sulfate reducing bacteria. Nat Rev Microbiol 6: 441-454.
- Laanbroek HJ, Stal LJ, Veldkamp H (1978) Utilization of hydrogen and formate by campylobacter spec. under aerobic and anaerobic conditions. Arch Microbiol 119: 99-102.
- Fujimoto D, Ishimoto M (1961) Sulfate reduction in Escherichia coli. J Biochem 50: 533-537.
- Tsang ML, Schiff JA (1976) Sulfate-reducing pathway in Escherichia coli involving bound intermediates. J Bacteriol 125: 923-933.
- Lautrop H, Orskov I, Gaarskev K (1979) Hydrogen sulphide producing variants of Escherichia coli. Acta Path Micro Im B 79: 641-650.
- Barbour EK, Nabbut NH, Al-Nakhli HM (1985) Production of H2S by Escherichia coli isolated from poultry: an unusual character useful for epidemiology of colisepticemia. Avian Dis 29: 341-346.
- Dreyfuss J (1964) Characterization of a sulfate- and thiosulfate-transporting system in Salmonella typhimurium. J Biol Chem 239: 2292-2297.
- Guarneros G, Ortega MV (1970) Cysteine desulfhydrase activities of Salmonella typhimurium and Escherichia coli. BBA 198: 132-142.
- Mallinson ET, Miller RG, Rezende CE, Ferris FE, deGraft-Hanson J, Joseph SW (2000) Improved plating media for the detection of salmonella species with typical and atypical hydrogen sulfide production. J Vet Diagn Invest 12: 83-87.
- Ishimoto M, Koyama J, Nagai Y (1955) Biochemical studies on sulfate-reducing bacteria. IV. Reduction of thiosulfate by cell-free extract. J Biochem 42: 41-53.
- Ishimoto M, Koyama J, Omura T, Nagai Y (1954) Biochemical studies on sulfate-reducing bacteria. III. Sulfate reduction by cell suspension. J Biochem 41: 537-546.
- Shrimpton DH (1966). Metabolism of the intestinal microflora in birds and its possible influence on the composition of flavor precursors in their muscle. J Appl Bacteriol 29: 222-230.
- Gong J, Forster RJ, Yu H, Chambers JR, Sabour PM, Wheatcroft R, et al. (2002) Diversity and phylogenetic analysis of bacteria in the mucosa of chicken ceca and comparison with bacteria in the cecal lumen. FEMS- Microbiol Lett 208: 1-7.
- Basic A, Blomqvist M, Dahlén G, Svensäter G (2017) The proteins of Fusobacterium spp. involved in hydrogen sulfide production from L-cysteine. BMC Microbiol 17: 61-70.
- Braunstein AE, Goryachenkova EV, Lac ND (1969) Reactions catalysed by serine sulfhydrase from chicken liver. BBA- Enzymol 171: 366-368.
- Sutton AL, Kephart KB, Verstegen MWA, Canh TT, Hobbs PJ (1999) Potential for reduction of odors compounds in swine manure through diet modification. J Anim Sci 77: 430-439.
- Kendall DC, Richert BT, Sutton AL, Bowers KA, Herr CT, et al. (2000) Effects of dietary manipulation on pig performance manure composition, hydrogen sulfide and ammonia levels in swine buildings. Purdue University Swine Day Reports.
- Jiao Y, Park JH, Kim YM, Kim IH (2017) Effects of dietary methyl sulfonyl methane (MSM) supplementation on growth performance, nutrient digestibility, meat quality, excreta microbiota, excreta gas emission, and blood profiles in broilers. Poult Sci pew 480.
- Bostami ABM, Mun HS, Kim DH, Yang CJ (2017) Evaluation of halal tallow and haram lard combinations on growth performance, immunity, cecal microbiology and noxious gas emissions in boilers. Int J Adv Res 4: 2376-2390.
- Bostami ABM, Mun HS, Kim GI, Seilsuth S, Yang CJ (2017) Evaluation of dietary fat sources on growth performance, excreta microbiology and noxious gas emissions in Ross broilers. Afr J Agr Res 12: 1980-1992.
- Sharma NK, Choct M, Wu SB, Smillie R, Swick RA (2015) Dietary composition affects odour emissions from meat chickens. Anim Nut 1: 24-29.
- Sharma NK, Choct M, Wu SB, Smillie R, Morgan N, et al. (2016) Performance, litter quality and gaseous odour emissions of broilers fed phytase supplemented diets. Anim Nut 2: 288-295.
- Wu-Haan W, Powers WJ, Angel CR, Hale-III CE, Applegate TJ (2007). Effect of an Acidifying Diet Combined with Zeolite and Slight Protein Reduction on Air Emissions from Laying Hens of Different Ages. Poult Sci 86: 182-190.
- Cai L, Koziel JA, Liang L, Nguyen AT, Xin H (2007). Evaluation of Zeolite for Control of Odorants Emissions from Simulated Poultry Manure Storage. J Environ Qual 36: 184-193.
- Wu-Haan W, Powers WJ, Angel CR, Applegate TJ (2010) The use of distillers dried grains plus solubles as a feed ingredient on air emissions and performance from laying hens. Poult Sci 89: 1355-1359.
- Ahmed ST, Kim G, Islam M, Mun HS, Bostami AB, Yang CJ (2015) Effects of dietary chlorine dioxide on growth performance, intestinal and excreta microbiology, and odorous gas emissions from broiler excreta. J. Appl. Poult Res 24: 502-510.
- Ferket PR, Heugten E, Kempen TATG, Angel R (2002) Nutritional strategies to reduce environmental emissions from nonruminants. J Anim Sci 80: E168-E182.
- Grashorn M (2010) Use of phytobiotics in broiler nutrition - An alternative to infeed antibiotics? J Anim Feed Sci 338-347.
- Panda K, Rama Rao SV, Raju MVLN (2006) Natural growth promoters have potential in poultry feeding systems. Feed Tech 10: 23-25.
- Li H, Zhao P, Lei Y, Hossain M, Kim I (2015) Phytoncide, phytogenic feed additive as an alternative to conventional antibiotics, improved growth performance and decreased excreta gas emission without adverse effect on meat quality in broiler chickens. Livest Sci 181: 1-6.
- Bostami ABM, Ahmed ST, Islam MM, Mun HS, Ko SY, Kim SS, et al. (2015) Growth Performance, Fecal Noxious Gas Emission and Economic Efficacy in Broilers Fed Fermented Pomegranate Byproducts as Residue of Fruit Industry. Int J Adv Res 3: 102-114.
- Ahmed ST, Yang CJ (2017) Effects of dietary Punica granatum l. by-products on performance, immunity, intestinal and fecal microbiology, and odorous gas emissions from excreta in Broilers. Poult Sci 54: 157-166.
- Jeong JS, Kim IH (2015) Effect of fermented medicinal plants (Gynura procumbens, Rehmannia glutinosa, Scutellaria baicalensis) as alternative performance enhancers in broilers. Jpn Poult Sci 52: 119-216.
- Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nut 125: 1401-1412.
- Gibson GR, Probert HM, Loo J, Rastall RA (2004) Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nut Res Rev 17: 259-275.
- Zhao PY, Wang JP, Kim IH (2014) Effect of dietary levan fructan supplementation on growth performance, meat quality, relative organ weight, cecal microflora, and excreta noxious gas emission in broilers. J Anim Sci 91: 5287-5293.
- Cho JH, Kim IH (2013) Effects of lactulose supplementation on performance, blood profiles, excreta microbial shedding of Lactobacillus and Escherichia coli, relative organ weight and excreta noxious gas contents in broilers. J Anim Physiol An N 98: 424-430.
- Fuller R (1989) Probiotics in man and animals. J Appl Bacteriol 66: 365-378.
- Fuller R (2001) The chicken gut microflora and probiotic supplements. Poult Sci 38: 189-196.
- Applegate TJ, Klose V, Steiner T, Ganner A, Schatzmayr S (2010) Probiotics and phytogenics for poultry: Myth or reality? Poult Sci Assoc Inc 19: 194-210.
- Naidu A, Xie X, Leumer D, Harrison S, Burrill M, et al. (2002) Reduction of sulfide, ammonia compounds, and adhesion properties of lactobacillus casei strain KE99 in vitro. Curr Microbiol 42: 196-205.
- Jeong JS, Kim IH (2014) Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poult Sci 93: 3097-3103.
- Jeong JS, Kim IH (2014) Effect of astaxanthin produced by Phaffia rhodozyma on growth performance, meat quality, and fecal noxious gas emission in broilers. Poult Sci 93: 3138-3144.
- Zhang ZF, Kim IH (2014) Effects of probiotic supplementation in different energy and nutrient density diets on performance, egg quality, excreta microflora, excreta noxious gas emission, and serum cholesterol concentrations in laying hens. J Anim Sci 91: 4781-4787.
- Lan RX, Lee SI, Kim IH (2017) Effects of Enterococcus faecium SLB 120 on growth performance, blood parameters, relative organ weight, breast muscle meat quality, excreta microbiota shedding, and noxious gas emission in broilers. Poult Sci 1-8.
- Zhang ZF, Cho JH, Kim IH (2013) Effects of Bacillus subtilis UBT-MO2 on growth performance, relative immune organ weight, gas concentration in excreta, and intestinal microbial shedding in broiler chickens. Livest Sci 155: 343-347.
- Sharma NK, Choct M, Dunlop MW, Wu SB, Castada HZ, Swick RA (2017) Characterisation and quantification of changes in odorants from litter headspace of meat chickens fed diets varying in protein levels and additives. Poult Sci 96: 851-860.
- Ahmed ST, Islam M, Mun HS, Sim HJ, Kim YJ, et al. (2014). Effects of Bacillus amyloliquefaciens as a probiotic strain on growth performance, cecal microflora, and fecal noxious gas emissions of broiler chickens. Poult Sci 93: 1963-1971.
- Mountzouris KC, Tsitrsikos P, Palamidi I, Arvaniti A, Mohnl M, et al. (2010) Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult Sci 89: 58-67.
- Chapman C, Gibson G, Rowland I (2011) Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nut 50: 1-17.
- Chang MH, Chen TC (2003) Reduction of broiler house malodor by direct feeding of a lactobacilli containing probiotic. Int J Poult Sci 2: 313-317.
- Fuchs AR, Bonde GE (1957) The availability of sulphur for Clostridium perfringens and an examination of hydrogen sulphide production. J Gen Microbiol 16: 330-340.
- Schostera A, Kokotovicb B, Perminc A, Pedersend P, Dal Bellod F, Guardabassia L (2013) In vitro inhibition of Clostridium difficile and Clostridium perfringens by commercial probiotic strains. Clin Microbiol 20: 36-41.
- Sharma NK, Keerqin C, Wu SB, Choct M, Swick RA (2017) Emissions of volatile odorous metabolites by Clostridium perfringens - in vitro studies using two broth cultures. Australian Poultry Science Symposium. Sydney, Australia. 28.
- Endo T, Nakano M (1999) Influence of a probiotic on productivity, meat components, lipid metabolism, caecal flora and metabolites, and raising environment in broiler production. J Anim Sci 70: 207-218.
- Zhang ZF, Kim IH (2014) Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poult Sci 93: 364-370.
- Balamuralikrishnan B, Lee SI, Kim IH (2017) Dietary inclusion of different multi-strain complex probiotics; effects on performance in broilers. Br Poult Sci 58: 83-86.
- Park JH, Kim IH (2015) The effects of the supplementation of Bacillus subtilis RX7 and B2A strains on the performance, blood profiles, intestinal Salmonella concentration, noxious gas emission, organ weight and breast meat quality of broiler challenged with Salmonella typhimurium. J Anim Physiol An N 99: 326-334.
- Hossain MM, Begum M, Kim IH (2015) Effect of Bacillus subtilis, Clostridium butyricum and Lactobacillus acidophilus endospores on growth performance, nutrient digestibility, meat quality, relative organ weight, microbial shedding and excreta noxious gas emission in broilers. Vet Med-Czech 60: 77-86.
- Lin XJ, Zhang R, Jiang S, Elmashad HM, Mitloehner F (2016) Nutrient flow and distribution in conventional cage, enriched colony, and aviary layer houses. Poult Sci 95: 213-224.
- Wu-Haan W, Powers WJ, Angel CR, Hale-III CE, Applegate TJ (2007) Nutrient digestibility and mass balance in laying hens fed a commercial or acidifying diet. Poult Sci 86: 684-690.
- Clanton CJ, Schmidt DR (2000) Sulfur compound in gases emitted from stored manure. Trans ASAE. 43: 1229-1239.
- Hacking A, Dervish MT, Rosser WR (1977) Available amino acid content and microbiological condition of dried poultry manure. Int J Poult Sci 18: 443-448.
- Hirai MF, Chanyasak V, Kubota H (1983) A standard measurement for compost maturity. BioCycle 24: 54-56.
- He ZQ, Olk DC (2011) Manure amino compounds and their bioavailability. Z.Q. He (Ed.), Environmental Chemistry of Animal Manure, Nova Science Publishers, New York. 179-199.
- Banwart WL, Bremner JM (1976) Evolution of volatile sulfur compounds from soils treated with sulfur-containing organic materials Soil Biol Biochem 8: 439-443.
- Smith K, Bremner JM, Tabatabai MA (1973) Sorption gaseous atmospheric pollutants by soils. Soil Sci 116: 313-319.
- Ghaly AE, MacDonald KN (2012) Drying of poultry manure for use as animal feed. Am J Agr Biol Sci 7: 239-254.
- Gay SW, Schmidt DR, Clanton CJ, Janni KA, Jacobson LD, Weisberg S (2003) Odor, total reduced sulfur, and ammonia emissions from animal housing facilities and manure storage units in Minnesota. Appl Eng Agr 19: 347-360.
- Xue SK, Chen S, Hermanson RE (1998) Measuring ammonia and hydrogen sulfide emitted from manure storage facilities. Am Soc Agr Eng 41: 1125-1130.
- Trudinger PA, Lambert IB, Skyring GW (1972) Biogenic sulfide ores: A feasibility study. Econ Geol 67: 1114-1127.
- Ludington DC, Sobel AT, Hashimoto AG (1971) Odors and Gases Liberated from Diluted and Undiluted Chicken Manure. Trans of the ASAE 14: 0855-0859.
- Sharma K, Derlon N, Hu S, Yuan Z (2014) Modeling the pH effect on sulfidogenesis in anaerobic sewer biofilm. Water Res 49: 175-185.
- Satoh H, Odagiri M, Ito T, Okabe S (2009) Microbial community structures and in situ sulfate-reducing and sulfur-oxidizing activities in biofilms developed on mortar specimens in a corroded sewer system. Water Res 43: 4729-4739.
- Choi HL, Richard TL, Ahn HK (2001) Composting high moisture materials: biodrying poultry manure in a sequentially fed reactor. Compost Sci Util 9: 303-311.
- Gutarowska B, Matusiak K, Borowski S (2014) Removal of odorous compounds from poultry manure by microorganisms on perlite-bentonite carrier. J Environ Manag 141: 70-76.
- Higgins MJ, Adams G, Chen YC, Erdal Z, Forbes Jr RH, Glindermann D, et al. (2008) Role of protein, amino acids, and enzyme activity on odor production from anaerobically digested and dewatered biosolids. Water Environ Res 80: 127-135.
- Matusiak K, Oleksy M, Borowski S, Nowak A, Korcvynski M, et al. (2016) The use of Yucca schidigera and microbial preparation for poultry manure deodorization and hygienization. J Environ Manag 170: 50-59.
- Cheeke P (2000) Actual and potential applications of and saponins in human and animal nutrition. J Anim Sci 77: 1-10.
- Borowski S, Matusiak K, PowaÃÆââ¬Â¦Ãâââ¬Å¡owski S, Pielech-Przybylska K, Makowski K, et al. (2017) A novel microbial-mineral preparation for the removal of offensive odors from poultry manure. Int Biodet Biodeg 119: 299-308.
- Kocaman B Yaganoglu AV, Yanar M (2005) Combination of fan ventilation system and spraying of oil-water mixture on the levels of dust and gases in caged layer facilities in Eastern Turkey. J Appl Anim Res 27: 109-111.
- Whitehead TR, Cotta MA, Spence C (2012) Application of tannins to reduce odor emissions from animal waste. United States Patent.
- NAEMS [Internet] National Air Emissions Monitoring Study.
- Lim TT, Heber AJ, Ni JQ (2003) Air quality measurements in a laying hen house: odor and hydrogen sulfide. Int. Symp. Gaseous Emissions Anim Prod Facil, Horsens, Denmark. CIGR, Bonn, Germany.
- Li H, Xin H, Burns RT, Roberts SA, Li S, Kliebenstein J, Bregendahl K (2012) Reducing ammonia emissions from laying-hen houses through dietary manipulation. J Air Waste Man 62: 160-169.
- Almuhanna EA, Ahmed AS, Al-Yousif YM (2011) Effect of Air Contaminants on Poultry Immunological and Production Performance. Int J Poult Sci 10: 461-470.
- Ni JQ, Diehl CA, Chai LL, Chen Y, Heber AJ, et al. (2017). Factors and characteristics of ammonia, hydrogen sulfide, carbon dioxide, and particulate matter emissions from two manure-belt layer hen houses. Atmos Environ 156: 113-124.
- Poultry housing A (2018) [Internet] Battery cage with manure-belt. Chore-time.
- Poultry housing B (2018) [Internet] High-rise with reverse stair-step. Indiamart. [cited 2018 Jan 6].
- Poultry housing C (2018) [Internet] Flat-deck. Unilio.
- Heber AJ, Ni JQ, Lim TT, Chervil R, Tao PC, et al. (2005) Aerial pollutant emissions from two high-rise layer barns in Indiana. A&WMA’s 98th Annual Conference and Exhibition. A&WMA, Pittsburgh, PA, Minneapolis, Minnesota.
- Lin XJ, Cortus EL, Zhang R, Jiang S, Heber AJ (2012) Ammonia, hydrogen sulfide, carbon dioxide and particulate matter emissions from California high-rise layer houses. Atmos Environ 46: 81-91.
- Wang K, Li Q, Li WL, Cortus E, Bogan BW, et al. (2016) National Air Emissions Monitoring Study's southeast layer site: Part V. Hydrogen sulfide and volatile organic compounds. Trans. ASABE 59: 681-690.
- Ni JQ, Liu S, Diehl CA, Lim TT, Bogan BW, et al. (2017) Emission factors and characteristics of ammonia, hydrogen sulfide, carbon dioxide, and particulate matter at two high-rise layer hen houses. Atmos Environ 154: 260-273.
- Kim KY (2016) Exposure level and emission characteristics of ammonia and hydrogen sulphide in poultry buildings of South Korea. Indoor and Built Environment 1-9.
- Leonard JJ, Feddes JJR, McQuitty JB (1983) Air quality in commercial broiler housing. Can Agr Eng 26: 65-71.
- McQuitty JB, Feddes JJR, Leonard JJ (1985) Air quality in commercial laying barns. Can Agr Eng 27: 13-19.
- Guarrasi J, Trask C, Kirychuk S (2015) A Systematic Review of Occupational Exposure to Hydrogen Sulfide in Livestock Operations. J Agromedicin 20: 225-236.
- Seppänen O, Kurnitski J (2018) [Internet] Moisture control and ventilation. In: WHO Guidelines for Indoor Air Quality: Dampness and Mould. Geneva: World Health Organization.
- Zhang G, Zhang Y, Kim Y, Kim J, Liu L, et al. (2011). Field study on the impact of indoor air quality on broiler production. Indoor Built Environ 20: 449-455.
- Schmidt D, Janni K, Nicolai R (2004) Biofilter Design Information. Biosystems and Agricultural Engineering Update. Department of Biosystems and Agricultural Engineering. University of Mennesota Extension Services.
- Chung YC, Huang C, Tseng CP (1996) Biodegradation of hydrogen sulfide by a laboratory-scale immobilized Pseudomonas putida ch11 biofilter. Biotectnol Progr 12: 773-778.
- Chung YC, Huang C, Tseng CP (1996) Operation optimization of Thiobacillus thioparus CH11 biofilter for hydrogen sulfide removal. J Biotechnol 52: 31-38.
- Chung YC, Huang C, Tseng CP, Pan JR (2000) Biotreatment of H2S- and NH3-containing waste gases by co-immobilized cells biofilter. Chemosphere 44: 329-336.
- Sercu B, NuNez D, Van Langenhove H, Aroca G, Verstraete W (2004) Operational and microbiological aspects of a bioaugmented two-stage biotrickling filter removing hydrogen sulfide and dimethyl sulfide. Biotechnol Bioeng 90: 259-269.
- Sun Y, Clanton CJ, Janni KA, Malzer GL (2000) Sulfur and Nitrogen balances in biofilters for odorous gas emission control. Am Soc Agr Eng
- Bohn H (1992) Consider biofiltration for decontaminating gases. Chem Eng Progr 88: 35-40.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences