In vitro, Evaluation of Aflatoxin B1 Binding by Strains of Probiotic Lactic Acid Bacteria, Lactobacillus reuteri DSM 20016T and Pediococcus pentosacus DSM 20206, Isolated from Chicken Gut
Kasech Melese*, Tesfaye Alemu, Asnake Desalegn, Bekisisa Urge and Abebe Olanne
Published Date: 2025-03-28Kasech Melese1*, Tesfaye Alemu2, Asnake Desalegn2, Bekisisa Urge3, Abebe Olanne4
1Ethiopian Institutes of Agricultural Research EIAR, Debre Zeit Agricultural Research Center, Addis Ababa, Ethiopia
2Department of Microbial, Cellular and Molecular Biology, Addis Ababa University, Addis Ababa, Ethiopia
3Ethiopian Institutes of Agricultural Research EIAR, Holeta Agricultural Research Center, Holeta, Ethiopia
4Department of Plant Science, Wollega University, Nekemte, Ethiopia
- Corresponding Author:
- Kasech Melese
Ethiopian Institutes of Agricultural Research EIAR, Debre Zeit Agricultural Research Center, Addis Ababa, Ethiopia
E-mail: kasechmelese@gmail.com
Received date: March 05, 2024, Manuscript No. IPJARN-24-18705; Editor assigned date: March 07, 2024, PreQC No. IPJARN-24-18705 (PQ); Reviewed date: March 21, 2024, QC No. IPJARN-24-18705;Revised date: March 21, 2025, Manuscript No. IPJARN-24-18705 (R); Published date: March 28, 2025, DOI: 10.36648/2572-5459.10.1.148
Citation: Melese K, Alemu T, Desalegn A, Urge B, Olanne A (2025) In vitro, Evaluation of Aflatoxin B1 Binding by Strains of Probiotic Lactic Acid Bacteria, Lactobacillus reuteri DSM 20016T and Pediococcus pentosacus DSM 20206, Isolated from Chicken Gut. J Anim Res Nutr Vol.10 No.1
Abstract
The potential of Lactic Acid Bacteria (LAB) strains to remove Aflatoxin B1 (AFB1) has been reported in previous studies. The present study evaluated the binding capabilities of Lactobacillus reuteri and Pediococcus pentosacus, which were isolated from the chicken gut. To assess the AFB1 binding abilities of these LAB strains, an in vitro experiment was conducted. The LAB strains were incubated with AFB1 (5 μg/mL) at a temperature of +37°C for one hour. After centrifugation, the supernatant fluids were analyzed using High-Performance Liquid Chromatography (HPLC) to quantify any unbound AFB1 remaining in the solution. The results of this in vitro experiment indicated that Lactobacillus reuteri DSM 20016T exhibited a binding rate of 55.28% for AFB1, while Pediococcus pentosacus DSM 20206 showed a binding rate of 36.24% in the supernatant. These findings highlight the potential of these LAB strains as probiotic candidates with AFB1 binding properties; further research and validation are needed to confirm their effectiveness in vivo.
Keywords
Aflatoxin B1; Binding; Chicken; In vitro; Lactic acid bacteria; Probiotic
Introduction
Aflatoxin B1 is a potent carcinogenic mycotoxin that is produced by certain species of fungi, such as Aspergillus flavus and Aspergillus parasiticus. It can contaminate various food and feed crops, particularly those that are improperly stored or exposed to high humidity and temperature conditions. Aflatoxin B1 has been implicated in the development of liver cancer and other health issues. Aflatoxin B1 is the most hepatotoxic, mutagenic and prevalent. Poultry products (meat and eggs) contaminated with aflatoxin have determinant effects on human health [1]. It is known to have harmful effects on various animals, including poultry. Aflatoxin B1 contamination in poultry feed can lead to several detrimental effects on poultry health and productivity. Aflatoxicosis in poultry is a health and overall performance threat that results in economic loss. Exposure to aflatoxin B1 leads to lower weight gain and feed intake, decreased feed efficiency, immunosuppression, as well as vaccination failures in chickens and turkeys, decreasing embryo viability and hatchability and reduced egg production and egg quality parameters [2].
It has been widely reported on a global scale that a significant portion of cereal crops are contaminated with mycotoxins, with aflatoxin being a major area of concern. The contamination of food products with mycotoxins results in substantial annual losses, estimated to be around 1 billion metric tons. In response to this issue, regulatory bodies like the US Food and Drug Administration have established guidelines for aflatoxin levels. Action levels for aflatoxin in animal feed, there are specific limits for aflatoxin in animal feed. For immature poultry, such as chicks, the safe limit for aflatoxin in corn and peanuts is set at 20 ppb. For mature poultry, the limit is slightly higher at 100 ppb. These guidelines help ensure the safety of animal feed and protect animal health.
According to the available literature, controlling aflatoxin contamination requires a multi-faceted approach involving various prevention and mitigation strategies. Here are some commonly employed aflatoxin control mechanisms: Good Agricultural Practices (GAPs): Pre-harvest and post-harvest management, pre-harvest and post-harvest management, chemical control, crop monitoring and testing and post-harvest processing storage conditions, regulatory measures and biological control: The use of biological control agents can help suppress aflatoxin-producing fungi. Certain non-toxic strains of Aspergillus spp. or other competitive fungi can be applied to crops to outcompete or inhibit the growth of aflatoxinproducing fungi.
According to a study conducted by Gizachew, et al. in the Greater Addis Ababa Milk Shed area of Ethiopia, the contamination levels of Aflatoxin B1 (AFB1) were investigated in individual samples of wheat bran and Noug cake [3]. The study found that the levels of AFB1 in wheat bran ranged from 9 to 31 μg/kg, while in Noug cake, the levels ranged from 290 to 397 μg/kg. These findings indicate the presence of aflatoxin contamination in these food products in the specified area. Furthermore, a recent report by Kassaw, et al. revealed the occurrence of aflatoxin in poultry feed samples collected in and around Bishoftu town, Ethiopia [4]. The study found that 66.67% of the poultry feed samples were contaminated with aflatoxin B1 and 72.75% of the samples were contaminated with total aflatoxins. These results highlight the presence of aflatoxin contamination in poultry feed in the specified region.
Lactic acid bacteria have shown the ability to bind and adsorb aflatoxin, including AFB1, to their cell surfaces. This binding capacity may help reduce the bioavailability of aflatoxin in the gastrointestinal tract, preventing its absorption into the bloodstream and subsequent toxicity. And also via enzymatic activity. Some LAB strains possess enzymatic activities, such as the production of hydrolytic enzymes, which can degrade or modify aflatoxin. These enzymes may break down the aflatoxin molecule into less toxic metabolites or completely degrade them, thereby reducing their harmful effects.
Lactobacillus reuteri is a species of lactic acid bacteria that is commonly found in the gastrointestinal tract of humans and other animals. It has been studied extensively for its potential health benefits, including its ability to inhibit the growth of pathogenic bacteria, modulate the immune system and improve gastrointestinal health.
In Ethiopia, there is an increasing demand for chicken probiotics as an alternative to antibiotics in poultry farming. This need arises from concerns regarding the use of antibiotics and the associated risks of aflatoxicosis in poultry production. However, the availability of commercial probiotics is limited and often requires costly imports. The objective of this study was to evaluate the ability of two specific strains, Lactobacillus reuteri (DSM 20016T) and Pediococcus pentosaccus (DSM 20206), which were isolated from the chicken gut, to bind aflatoxin B1 in vitro.
Materials and Methods
Bacterial strain and culture condition
The LAB strains used in this study were obtained from a previous investigation and were initially evaluated for their potential probiotic characteristics. These characteristics included antimicrobial activities, tolerance to low acid pH and 0.3% bile salt and adhesion to chicken intestinal epithelial cells. The specific strains selected for their aflatoxin B1 binding ability were Lactobacillus reuteri DSM 20016T and Pediococcus pentosaccus DSM 20206. To identify and confirm the strains, Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight (MALDI-TOF) mass spectrometry was employed. The obtained strains were compared to the Bruker MALDI Biotyper database for strain identification. To cultivate the LAB strains, de Man, Rogosa and Sharpe (MRS) agar was used and the cultures were incubated at a temperature of +37°C for 24-48 hours under anaerobic conditions in an anaerobic jar [5]. In order to ensure purity, the cultures were purified by re-streaking on MRS agar and sub-culturing in MRS broth before each evaluation step.
Aflatoxin B-1 binding assay
To prepare the Aflatoxin B1 (AFB1) standard solution, benzene-acetonitrile was utilized, resulting in an approximate concentration of 5 μg/mL. For the aflatoxin B1 binding experiment, a 5 μg/mL AFB1 standard solution was prepared in PBS (pH 7.3). The benzene-acetonitrile solvent was removed by heating the solution in a water bath at +70°C for 10 minutes. The LAB strains were subcultured from the MRS slant agar to fresh MRS broth (Himedia) and incubated for 24 hours at +37°C under anaerobic conditions, following the method described by de Man, Rogosa and Sharpe. To estimate the bacterial cell concentrations in the culture, serial dilutions were performed using Phosphate-Buffered Saline (PBS). To avoid interference from MRS broth, the bacterial cell concentrations, approximately 1 × 108 Colony Forming Units (CFU), were washed twice with Phosphate Buffer Saline (PBS) before initiating the binding process. By washing the LAB strains with PBS, any potential influence of MRS broth on the bacterial concentration during the binding process was minimized. This ensured that the subsequent binding experiments accurately reflected the interaction between the LAB strains and aflatoxin B1.
The bacterial cultures were centrifuged using a Thermo Scientific Pico 21 centrifuge at 6000 rpm for 15 minutes at 10°C. After centrifugation, the bacterial cell pellets were obtained and suspended in 1.0 ml of PBS containing the AFB1 solution at a concentration of 5 μg/ml. The suspension was then incubated for one hour at +37°C. After incubation, samples of the supernatant fluid (100 μl) containing any unbound AFB1 were collected and stored at -20°C for subsequent analysis using High- Performance Liquid Chromatography (HPLC). This analysis would help determine the amount of AFB1 that remained unbound after the interaction with the LAB strains. To serve as controls, both a bacterial control (bacteria suspended in PBS) and an AFB1 control (containing 5 μg/ml of AFB1 in PBS) were incubated under the same conditions as the experimental samples. These controls were necessary for comparison and to assess the binding capacity of the LAB strains.
Quantitation of residual aflatoxin B-1
High-Performance Liquid Chromatography (HPLC) is a widely used technique for analyzing aflatoxin in feeds and foodstuffs. It employs various detection methods such as fluorescence, UV absorption, mass spectrophotometry and amperometric detectors. In HPLC, a liquid mobile phase carries the sample through a column containing an immobilized liquid stationary phase. The analyte is detected as it passes through the column based on differences in partition coefficients [6].
In this study, the supernatant fluid samples were analyzed for Aflatoxin B1 (AFB1) residues using a reverse-phase HPLC method that did not require a sample extraction step. The HPLC system consisted of a dual-pump solvent delivery system (Waters e2695 module), a programmable Fluorescence Detector (FLD 2475) and a 4.6 mm × 250 mm (5 μm) eclipse plus C 18 column equipped with a guard column. The autoinjector sample injection volume was set to 10 μl. AFB1 was eluted isocratically using a mobile phase of MilliQ-water/acetonitrile/methanol (60/15/25; vol/vol/ vol) at a flow rate of 1 ml/min. The excitation and emission wavelengths for detection were set at 365 nm and 435 nm, respectively. The retention time for AFB1 was approximately 14 minutes. The aflatoxin analysis was conducted using HPLC at the Ethiopian Conformity Assessment Enterprise in Addis Ababa, Ethiopia. To determine the percentage of aflatoxin B1 binding in the assays, the following formula was used: The percentage of aflatoxin B1 binding in the assays can be calculated using the following formula:
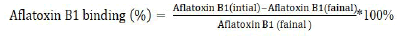
Statistical analysis
The percentage of AFB1 binding for each bacteria was determined. The experiment was carried out in duplicate and the High Performance Liquid Chromatography (HPLC) method was used to analyze the binding of AFB1 by the bacterial strains.
Results
Aflatoxin B1 binding assay
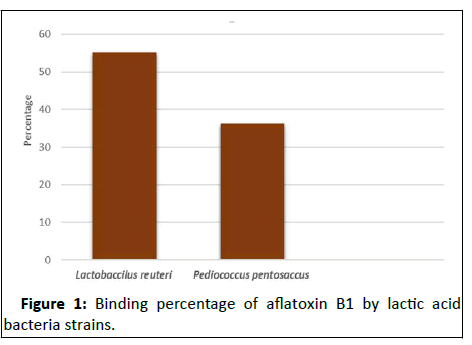
The aflatoxin B1-binding capacity of LAB isolates was examined; Lactobacillus reuteri DSM 20016T was recorded with binding rates of 55.28% and Pediococcus pentosacus DSM 20206 with 36.24% (Figure 1).
Figure 1: Binding percentage of aflatoxin B1 by lactic acid bacteria strains.
Discussion
The objective of this study was to investigate the ability of lactobacillus and Pediococcus strains to bind aflatoxin B1 in vitro. Probiotics that exhibit strong mycotoxin-binding capabilities hold promising potential as agents for mycotoxin detoxification. The prevailing theory regarding the mechanism of Lactic Acid Bacteria (LAB) binding to mycotoxins suggests that it occurs through physical adsorption between the mycotoxin molecules and the cell wall of the microorganisms. These interactions are presumed to be non-covalent and rely on van der Waals forces, hydrophobic interactions and hydrogen bonding. Previous speculation has suggested that the composition of cell wall polysaccharides and peptidoglycans may influence the binding process.
In our study, we evaluated the ability of Lactobacillus reuteri (DSM 20016T) and Pediococcus pentosaccus (DSM 20206) to bind aflatoxin B1 and observed different binding percentages for each strain. These differences can likely be attributed to variations in the bacterial cell wall composition and overall bacterial cell structures. To determine the appropriate aflatoxin B1 binding time for our investigation, we referred to previous research that aimed to establish the duration of contact between AFB1 and bacteria. It was found that altering the incubation duration did not result in any noticeable variation in the amount of AFB1 eliminated by the LAB and yeast strains. This led us to select a binding time of 1 hour for the present study. Moreover, the binding process was found to be rapid, as the microbes were able to bind the same quantity of mycotoxin in just 1 minute as they did in 6 hours. By considering the specific strains of bacteria, their cell wall characteristics and the optimal binding time, we were able to assess the binding capacity of L. reuteri and P. pentosaccus towards aflatoxin B1 in our study.
Afshar, et al. conducted a study on probiotic lactic acid bacteria and reported aflatoxin binding rates ranging from 9% to 100% [7]. This indicates that different strains of lactic acid bacteria can exhibit varying abilities to bind aflatoxin. Atehnkeng, et al. identified a toxigenic fungal species naturally present in the environment that competes with toxin-producing fungi [8]. This finding suggests that there are natural mechanisms in the environment that can help mitigate the presence of mycotoxins. El-Nezami, et al. investigated the efficacy of lactic acid bacteria strains in removing Fusarium toxins from suspensions [9]. They found that strains such as Lac. rhamnosus GG, Lac. rhamnosus LC-705 and Propionibacterium freudenreichii JS (PJS) were able to remove Fusarium toxins with removal percentages ranging from 18% to 93%. This highlights the potential of certain lactic acid bacteria strains in mycotoxin removal.
Similarly, Liew, et al. reported that live cells of Lactobacillus showed a high binding capacity of 98% to aflatoxin B1 [10]. Microscopy tests conducted in their study also demonstrated changes in the bacterial cell surface upon binding to aflatoxin. These microscopy observations provided solid visual proof of the interaction between bacteria and aflatoxin. The findings from both studies emphasize the significant role of binding interactions between bacteria and aflatoxin. The microscopy experiments not only confirmed the binding but also highlighted the impact of this interaction on the bacterial cell surface. Such insights contribute to our understanding of the mechanisms underlying the interactions between bacteria and mycotoxins, specifically aflatoxin B1.
In a study by Peltonen, et al. various strains of Lactobacillus, Bifidobacterium and Lactococcus were examined for their ability to bind aflatoxin B1 [11]. The binding rates ranged from 5.6% to 59.7% of aflatoxin B1 from the solution, as quantified by HPLC. Sellamani, et al. investigated the antifungal capability of Pediococcus pentosaccus isolated from dairy products [12]. They found that it exhibited antifungal activity by reducing the mycelial biomass, with a zone of inhibition measured at 47.1 ± 2.81%.
A Hamidi, et al. isolated strains of Lactobacillus pentosus and Lactobacillus beveris from human feces and milk samples, respectively [13]. They found that these strains removed 17.4% and 34.7% of aflatoxin B1 from the suspension, respectively. Damayanti, et al. observed the highest aflatoxin reduction with viable cells of Lactobacillus plantarum [14]. The binding rate for this strain was 69.11%. A systematic review and meta-analysis conducted by Alireza, et al. reported the binding rates of different lactic acid bacteria strains in reducing aflatoxin B1 [15 16]. They found Lactobacillus to have a binding rate of 47.96%, Bifidobacterium 43.95%, Pediococcus 41.61%, Lactococcus 33.56% and Enterococcus 27.14%. A recent study by Mosallaie, et al. evaluated the ability of two strains of lactic acid bacteria and a yogurt starter culture to bind AFB1 [16]. They observed high binding capacities in all evaluated treatments, ranging from 64.56% to 96.58%. Probiotic bacteria, however, exhibited statistically superior binding capacity compared to yogurt starter cultures. A study by Escrivá, et al. found that seven LABs were able to reduce AFB1 levels by 11-35% [17]. Notably, Lactobacillus plantarum B3 exhibited the highest activity in reducing AFB1. Byakika, et al. reported LAB-bound 19.3%-69.4% of AFB1 in the solution of sorghum-millet beverages [18]. In a study conducted by Martinez, et al. they investigated the adsorption capability of different strains of Lactic Acid Bacteria (LAB) to degrade Aflatoxin B1 (AFB1) [19]. Among the strains tested, Pediococcus acidilactici RC003 exhibited the most promising results, with a degradation rate of over 15% in vitro. Ramo, et al. demonstrated that L. plantarum as a starter culture in the fermentation of a plant-based protein-rich food product effectively reduces the amount of free aflatoxin B1 as high as 90% [20].
Conclusion
LAB cultures with strong mycotoxin binding capacities and probiotic abilities are of enormous value in lowering aflatoxin exposure, especially given the growing concern about food safety and aflatoxicosis in poultry. The study implies that L. reuteri (DSM 20016T) and P. pentosaceus (DSM 20206) are promising potential probiotic strains with good aflatoxin B1- binding properties in vitro. These findings highlight the significance of L. reuteri and P. pentosaccus in mitigating the risks associated with AFB1 exposure in poultry production, ultimately contributing to improved food safety and human health. Further research and exploration are needed to better understand the underlying mechanisms of AFB1 degradation by this LAB strain and in vivo aflatoxin decontamination activity.
Acknowledgments
We acknowledge the Department of Microbial, Cellular and Molecular Biology and the Food Microbiology Laboratory for their cooperation and technical assistance during this project. And also the Ethiopian Institute of Agriculture Research, Debre Zeit Center, for their approval and support of this research.
Funding
The Ethiopian Institutes of Agricultural Research and the Addis Ababa University College of Natural and Computational Science provided funding for this study. The sponsoring organization got a quarterly report and frequently followed the development of the study, offering helpful advice and ideas.
Author Contributions
K.M., T.A., A.D., and B.U. designed the study. K.M. sample collection and laboratory work. K.M., A.D., and A.O. analyzed the results and all the participating authors read and approved the submitted manuscript.
Data Availability
The data supporting the findings of this study are available on request to the corresponding author.
Conflict of Interest
The authors declared that they have no conflicts of interest.
References
- Ahmad S, Anwar S, Pratap PD (2022) The characteristic, occurrence of aflatoxin and associated risk with human health. Microbiol Res J Int 32:39-50
- Pandey I, Chauhan SS (2007) Studies on production performance and toxin residues in tissues and eggs of layer chickens fed on diets with various concentrations of aflatoxin AFB1. Br Poult Sci 48:713-723
[Crossref] [Google Scholar] [PubMed]
- Gizachew D, Szonyi B, Tegegne A, Hanson J, Grace D (2016) Aflatoxin contamination of milk and dairy feeds in the Greater Addis Ababa milk shed, Ethiopia. Food Control 59:773-779
- Kassaw TS, Megerssa YC, Woldemariyam FT (2022) Occurrence of aflatoxins in poultry feed in selected chicken rearing villages of Bishoftu Ethiopia. Vet Med 13:277-286
[Crossref] [Google Scholar] [PubMed]
- Jose NM, Bunt CR, Hussain MA (2015) Comparison of microbiological and probiotic characteristics of lactobacilli isolates from dairy food products and animal rumen contents. Microorganisms 3:198-212
[Crossref] [Google Scholar] [PubMed]
- Coskun O (2016) Separation techniques: Chromatography. North Clin Istanb 3:156-160
[Crossref] [Google Scholar] [PubMed]
- Afshar P, Shokrzadeh M, Raeisi SN, Ghorbani-HasanSaraei A, Nasiraii LR (2020) Aflatoxins biodetoxification strategies based on probiotic bacteria. Toxicon 178:50-58
[Crossref] [Google Scholar] [PubMed]
- Atehnkeng J, Donner M, Ojiambo PS, Ikotun B, Augusto J, et al. (2016) Environmental distribution and genetic diversity of vegetative compatibility groups determine biocontrol strategies to mitigate aflatoxin contamination of maize by Aspergillus flavus. Microb Biotechnol 9:75-88
[Crossref] [Google Scholar] [PubMed]
- El-Nezami HS, Chrevatidis A, Auriola S, Salminen S, Mykkänen H (2002) Removal of common Fusarium toxins in vitro by strains of Lactobacillus and Propionibacterium. Food Addit Contam 19:680-686
[Crossref] [Google Scholar] [PubMed]
- Liew WP, Nurul-Adilah Z, Than LT, Mohd-Redzwan S (2018) The binding efficiency and interaction of Lactobacillus casei Shirota toward aflatoxin B1. Front Microbiol 9:1503
[Crossref] [Google Scholar] [PubMed]
- Peltonen K, El-Nezami H, Haskard C, Ahokas J, Salminen S (2001) Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J Dairy Sci 84:2152-2156
[Crossref] [Google Scholar] [PubMed]
- Sellamani M, Kalagatur NK, Siddaiah C, Mudili V, Krishna K, et al. (2016) Antifungal and zearalenone inhibitory activity of Pediococcus pentosaceus isolated from dairy products on Fusarium graminearum. Front Microbiol 7:890
[Crossref] [Google Scholar] [PubMed]
- Hamidi A, Mirnejad R, Yahaghi E, Behnod V, Mirhosseini A, et al. (2013) The aflatoxin B1 isolating potential of two lactic acid bacteria. Asian Pac J Trop Biomed 3:732-736
[Crossref] [Google Scholar] [PubMed]
- Damayanti E, Istiqomah L, Saragih JE, Purwoko T (2017) Characterization of lactic acid bacteria as poultry probiotic candidates with aflatoxin B1 binding activities. IOP Conf Ser Earth Environ Sci 101:012030
- Emadi A, Eslami M, Yousefi B, Abdolshahi A (2022) In vitro strain specific reducing of aflatoxin B1 by probiotic bacteria: A systematic review and meta-analysis. Toxin Rev 41:995-1006
- Mosallaie F, Jooyandeh H, Hojjati M, Fazlara A (2020) Biological reduction of aflatoxin B1 in yogurt by probiotic strains of Lactobacillus acidophilus and Lactobacillus rhamnosus. Food Sci Biotechnol 29:793-803
[Crossref] [Google Scholar] [PubMed]
- Escrivá L, Calpe J, Lafuente C, Moreno A, Musto L, et al. (2023) Aflatoxin B1 and ochratoxin A reduction by Lactobacillus spp. during bread making. J Sci Food Agric 103:7095-7103
[Crossref] [Google Scholar] [PubMed]
- Byakika S, Mukisa IM, Wacoo AP, Kort R, Byaruhanga YB, et al. (2019) Potential application of lactic acid starters in the reduction of aflatoxin contamination in fermented sorghum-millet beverages. Int J Food Contam 6:4
- Martinez MP, Pereyra MLG, Pena GA, Poloni V, Juri GF, et al. (2017) Pediococcus acidolactici and Pediococcus pentosaceus isolated from a rainbow trout ecosystem have probiotic and ABF1 adsorbing/degrading abilities in vitro. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 34:2118-2130
[Crossref] [Google Scholar] [PubMed]
- Rämö S, Kahala M, Joutsjoki V (2022) Aflatoxin B1 binding by lactic acid bacteria in protein-rich plant material fermentation. Appl Sci 12: 12769

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences